��Ŀ����
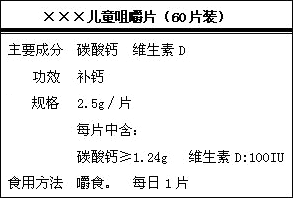
��ͼ�ǡ���������Ƭ��Ʒ��ǩͼ��
| ��������ͯ��Ƭ ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l.U. [ʳ�÷���]��ʳ,ÿ��һƬ [��Ч]���� |
���ݱ�ǩ������Ϣ������ش���������: �������ȷ��0.1��
��1�� ��Ҫ�ɷ�̼����и�Ԫ�ص���������Ϊ______________��ÿƬ�����ٺ���Ԫ�ص�����Ϊ______________________g��
��2�� С��ͬѧΪ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4Ƭ��Ƭ�����������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ����Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ���ϡ���ᷴӦ����
�Լ��㣺�����ɶ�����̼������____________��
��ϡ���������ʵ���������____________��
��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ________________��
��1��40% ��1�֣� 0.5 ��1�֣�
��2���ٸ��������غ㶨�ɿ�֪�����ɶ�����̼������Ϊ��2.2g ��1�֣�
��ϡ���������ʵ���������Ϊ9.13%����2�֣�
��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע��ʵ����1�֣�

��1����Ҫ�ɷ�̼��Ƶ���Է�������Ϊ
��2��С��ͬѧΪ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4ƬƬ�������������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ�����ʵ�����Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ��������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�����������
�����ɶ�����̼��������
��ϡ���������ʵ�������������ȷ��0.01����
��3��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
| ��������ͯ��Ƭ ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l��U�� [ʳ�÷���]��ʳ��ÿ��һƬ [��Ч]���� |
| ��������ͯ��Ƭ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l��U�� [ʳ�÷���]��ʳ��ÿ��һƬ [��Ч]���� |
��1����Ҫ�ɷ�̼����и�Ԫ�ص���������Ϊ
��2��С��ͬѧΪ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4Ƭ��Ƭ�����������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ����Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ���ϡ���ᷴӦ�����Լ��㣺�����ɶ�����̼��������
��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ����Ҫ���м�����̣�
��ͼ�ǡ�����������Ƭ��Ʒ��ǩͼ�����ܸ��ݱ�ǩ��Ϣ������������𣿣�
��1����Ҫ�ɷ�̼��Ƶ���Է�������Ϊ______��ÿƬ�����ٺ���Ԫ�ص�����Ϊ______g����������λ��Ч���֣�
��2��С��ͬѧΪ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4ƬƬ�������������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ�����ʵ�����Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ��������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�����������
�����ɶ�����̼��������
��ϡ���������ʵ�������������ȷ��0.01����
��3��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
| ��������ͯ��Ƭ ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l��U�� [ʳ�÷���]��ʳ��ÿ��һƬ [��Ч]���� |