��Ŀ����
��ͼ�ǡ�����������Ƭ��Ʒ��ǩͼ�����ܸ��ݱ�ǩ��Ϣ������������𣿣�
��1����Ҫ�ɷ�̼��Ƶ���Է�������Ϊ______��ÿƬ�����ٺ���Ԫ�ص�����Ϊ______g����������λ��Ч���֣�
��2��С��ͬѧΪ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4ƬƬ�������������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ�����ʵ�����Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ��������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�����������
�����ɶ�����̼��������
��ϡ���������ʵ�������������ȷ��0.01����
��3��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
| ��������ͯ��Ƭ ��60Ƭװ�� |
| [��Ҫ�ɷ�]̼��ƣ�ά����D [���]2.5g/Ƭ��ÿƬ�к�̼��ơ�1.24g��ά����D 100l��U�� [ʳ�÷���]��ʳ��ÿ��һƬ [��Ч]���� |
�⣺��1��̼��ƵĻ�ѧʽΪ��CaCO3����̼��Ƶ���Է�������Ϊ��40+12+16��3=100��
̼��ƣ�CaCO3���и�Ԫ�ص���������Ϊ�� =40%��
=40%��
��ÿƬ�����ٺ���Ԫ�ص�����Ϊ1.24g��40%=0.50g��
�ʴ�Ϊ��100��0.50��
��2���⣺�ٸ��������غ㶨�ɿ�֪������CO2������Ϊ��40g+4��2.5g-47.8g=2.2g
����μӷ�Ӧ��CaCO3��HCl�������ֱ�Ϊx��y
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 2.2g

���y=3.65g
��ϡ���������ʵ���������Ϊ�� ��100%=9.13%��
��100%=9.13%��
��3��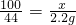 ��
��
��ã�x=5g��
ÿƬ��CaCO3������Ϊ�� =1.25g��1.24g���ʸ�Ƭ��̼��Ƶĺ�����עȷ��
=1.25g��1.24g���ʸ�Ƭ��̼��Ƶĺ�����עȷ��
�����ɶ�����̼��������2.2g��ϡ���������ʵ���������Ϊ9.13%��ͨ�����㺬����ע��ʵ��
��������1������̼��ƵĻ�ѧʽ��������Է����������ڸ�Ԫ�����ԭ�������ĺͼ������̼��Ƶ���Է�����������Ԫ�ض���̼������ˣ���̼��Ƶ���������Ԫ����̼����е����������������Ԫ�ص�������
��2���������������غ㣬��������ɵĶ�����̼���������ٸ��ݻ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ�����������Ӧ��HCl���������ٸ���������������=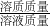 ��100%�������ϡ���������ʵ�����������
��100%�������ϡ���������ʵ�����������
��3�����ݣ�2���еķ���ʽ�Ļ����Լ����4Ƭ��Ƭ��̼��Ƶ����������������һƬ��Ƭ��̼��Ƶ������������ǩ���Ƚϼ��ɣ�
������������Ҫ����ѧ�����û�ѧʽ�Լ���ѧ����ʽ���н��м�����������������Ĺؼ��Ǽ������ɶ�����̼��������
̼��ƣ�CaCO3���и�Ԫ�ص���������Ϊ��
 =40%��
=40%����ÿƬ�����ٺ���Ԫ�ص�����Ϊ1.24g��40%=0.50g��
�ʴ�Ϊ��100��0.50��
��2���⣺�ٸ��������غ㶨�ɿ�֪������CO2������Ϊ��40g+4��2.5g-47.8g=2.2g
����μӷ�Ӧ��CaCO3��HCl�������ֱ�Ϊx��y
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 2.2g

���y=3.65g
��ϡ���������ʵ���������Ϊ��
 ��100%=9.13%��
��100%=9.13%����3��
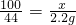 ��
����ã�x=5g��
ÿƬ��CaCO3������Ϊ��
 =1.25g��1.24g���ʸ�Ƭ��̼��Ƶĺ�����עȷ��
=1.25g��1.24g���ʸ�Ƭ��̼��Ƶĺ�����עȷ�������ɶ�����̼��������2.2g��ϡ���������ʵ���������Ϊ9.13%��ͨ�����㺬����ע��ʵ��
��������1������̼��ƵĻ�ѧʽ��������Է����������ڸ�Ԫ�����ԭ�������ĺͼ������̼��Ƶ���Է�����������Ԫ�ض���̼������ˣ���̼��Ƶ���������Ԫ����̼����е����������������Ԫ�ص�������
��2���������������غ㣬��������ɵĶ�����̼���������ٸ��ݻ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ�����������Ӧ��HCl���������ٸ���������������=
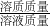 ��100%�������ϡ���������ʵ�����������
��100%�������ϡ���������ʵ�������������3�����ݣ�2���еķ���ʽ�Ļ����Լ����4Ƭ��Ƭ��̼��Ƶ����������������һƬ��Ƭ��̼��Ƶ������������ǩ���Ƚϼ��ɣ�
������������Ҫ����ѧ�����û�ѧʽ�Լ���ѧ����ʽ���н��м�����������������Ĺؼ��Ǽ������ɶ�����̼��������

��ϰ��ϵ�д�
 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ
?��ͼ�ǡ���������������Ʒ��ǩͼ������ݱ�ǩ��Ϣ����������⣺
��1��ÿƬҩ�������ٺ���Ԫ�ص�����Ϊ g��
��2��С��Ϊ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4ƬƬ�������������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ��������Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ����ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
| ��������ͯ��Ƭ ��60Ƭװ�� [��Ҫ�ɷ�]̼��ơ�ά����D |
| [��Ч]���� [���]25g/Ƭ ÿƬ�к��� ̼��ơ�124g ά����D100IU [ʳ�÷���]��ʳ��ÿ��1Ƭ |
��2��С��Ϊ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4ƬƬ�������������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ��������Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ����ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��



