��Ŀ����
��ͼ�ǡ�����������Ƭ��Ʒ��ǩͼ��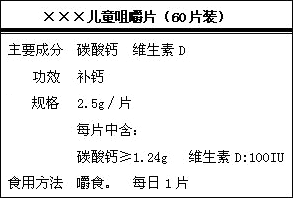
��1����Ҫ�ɷ�Ϊ̼��Ƶ���Է�������Ϊ���٣�ÿƬ�����ٺ���Ԫ�ص�����Ϊ���ٿˣ�
��2��С��ͬѧΪ�˲ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��6ƬƬ�������������ձ��У�����ϡ���ᣬ�����ٲ�������Ϊֹ������ȥϡ����40g�������ձ���ʣ����������Ϊ51.7g����Ƭ�������ɷֲ���ϡ���ᷴӦ��
�������ɶ�����̼��������
��ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ�������ԭ������ Ca-40 C-12 O-16��
���������ʵ���Է���������ָ���Ӹ���ԭ�ӵ����ԭ�������ĺͣ�ij������ijԪ�ص�����=�����ʵ���������Ԫ���������е��������������������غ㶨�ɾͿ�������ɵ���������������ʵ��������ļ��ٵ�����Ȼ������ɵ�������������뻯ѧ����ʽ�Ϳ������ص�����
����⣺��1��̼��Ƶ���Է�������Ϊ��40+12+16��3=100
ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ1.24g��
��100%=0.496g
��2�����������غ㶨�ɵã����ɵĶ�����̼������Ϊ��2.5g��6+40g-51.7g=3.3g
��6Ƭ��Ƭ��������̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.3g
=
x=
=7.5g
��ÿƬ��Ƭ��̼��Ƶ�����Ϊ
=1.25g��1.24g
�ʸ�Ƭ��̼��Ƶĺ�����ע����ʵ��
�𣺣�1��̼��Ƶ���Է���������100��ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ0.496g
��2�����ɵĶ�����̼������Ϊ3.3g��ͨ������ø�Ƭ��̼��Ƶĺ�����ע����ʵ�ģ�
ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ1.24g��
| 40 |
| 100 |
��2�����������غ㶨�ɵã����ɵĶ�����̼������Ϊ��2.5g��6+40g-51.7g=3.3g
��6Ƭ��Ƭ��������̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.3g
| 100 |
| 44 |
| x |
| 3.3g |
x=
| 100��3.3g |
| 44 |
��ÿƬ��Ƭ��̼��Ƶ�����Ϊ
| 7.5g |
| 6 |
�ʸ�Ƭ��̼��Ƶĺ�����ע����ʵ��
�𣺣�1��̼��Ƶ���Է���������100��ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ0.496g
��2�����ɵĶ�����̼������Ϊ3.3g��ͨ������ø�Ƭ��̼��Ƶĺ�����ע����ʵ�ģ�
�����������ѶȲ��Ǻܴ���Ҫ�������йػ�ѧʽ�ļ��㡢�����غ㶨�ɵ�Ӧ�ú��ݻ�ѧ����ʽ������صļ��㣮

��ϰ��ϵ�д�
 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ
?��ͼ�ǡ���������������Ʒ��ǩͼ������ݱ�ǩ��Ϣ����������⣺
��1��ÿƬҩ�������ٺ���Ԫ�ص�����Ϊ g��
��2��С��Ϊ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4ƬƬ�������������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ��������Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ����ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��
| ��������ͯ��Ƭ ��60Ƭװ�� [��Ҫ�ɷ�]̼��ơ�ά����D |
| [��Ч]���� [���]25g/Ƭ ÿƬ�к��� ̼��ơ�124g ά����D100IU [ʳ�÷���]��ʳ��ÿ��1Ƭ |
��2��С��Ϊ�ⶨ��̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��4ƬƬ�������������ձ��У���μ���ϡ���ᣬ�����ٷų�����Ϊֹ������ȥϡ����40.0g�������ձ���ʣ��������Ϊ47.8g�������ձ��������ٶ���Ƭ�����ɷֲ������ᷴӦ����ͨ�������жϸ�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ��



