��Ŀ����

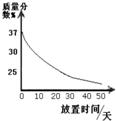
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ1������ˮ������������Ũ������������������仯��ԭ��
Ũ����ӷ��������Ȼ���
Ũ����ӷ��������Ȼ���
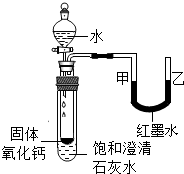
����2���۲�ͼ2����С�Թ��ڵμ�ˮ�ɹ۲쵽��������
ʯ��ˮ����ǣ���Һ���½����Ҷ�����
ʯ��ˮ����ǣ���Һ���½����Ҷ�����
�����Ͳ����������ԭ����ʯ����ˮ��Ӧ�ų�������ʹ�¶����ߣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ��Թ��ڵ�ѹǿ����
��ʯ����ˮ��Ӧ�ų�������ʹ�¶����ߣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ��Թ��ڵ�ѹǿ����
����3��ͼ3ͼ4�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ��
����
����
������ԡ�?�����ԡ����ԡ���ͬ�����Ҵ���ˮ��Һ������
����
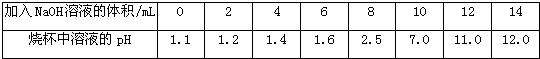
����4����ʢ��10mLϡ���ᣨ���е�������ָʾ����ɫ��̪�����ձ��м�������������Һ���������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ��
NaCl
NaCl
���������������е�֪ʶ���з�����Ũ������лӷ��ԣ��ӷ��������Ȼ��⣬����������ˮ��Ӧ�����������Ʋ��ų��������ȣ�����ָ����ʱ�γɵ�������ȫ���������ӵĻ������̪��Һ�����Ի�������Һ��Ϊ��ɫ��
����⣺��1��Ũ������лӷ��ԣ��ӷ��������Ȼ��⣬���ʼ��٣��ܼ����䣬����Һ��ϡ����������������С�����Ũ����ӷ��������Ȼ��⣻
��2����������ˮ��Ӧ������������ͬʱ�ų��������ȣ��¶����ߣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ��ʱ���ʯ��ˮ���������ʶ�����ǣ��¶��������Թ��ڵ�ѹǿ������U�ι��ڼ�Һ���½����Ҷ����������ʯ��ˮ����ǣ���Һ���½����Ҷ���������ʯ����ˮ��Ӧ�ų�������ʹ�¶����ߣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ��Թ��ڵ�ѹǿ����
��3����ͼ��֪���廯���ˮ��Һ�е�������ȫ���������ӣ���Һ�����ԣ����Ҵ���ˮ��Һ��û�������ӻ����������ӣ������ԣ�������ԣ����ԣ�
��4�����з�̪��������Һ�м�������������Һ����ҺΪ��ɫ��˵����Һ�����Ի����ԣ����������������ᷴӦ�����Ȼ��ƺ�ˮ������Һ��һ���е��������Ȼ��ƣ����NaCl��
��2����������ˮ��Ӧ������������ͬʱ�ų��������ȣ��¶����ߣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ��ʱ���ʯ��ˮ���������ʶ�����ǣ��¶��������Թ��ڵ�ѹǿ������U�ι��ڼ�Һ���½����Ҷ����������ʯ��ˮ����ǣ���Һ���½����Ҷ���������ʯ����ˮ��Ӧ�ų�������ʹ�¶����ߣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ��Թ��ڵ�ѹǿ����
��3����ͼ��֪���廯���ˮ��Һ�е�������ȫ���������ӣ���Һ�����ԣ����Ҵ���ˮ��Һ��û�������ӻ����������ӣ������ԣ�������ԣ����ԣ�
��4�����з�̪��������Һ�м�������������Һ����ҺΪ��ɫ��˵����Һ�����Ի����ԣ����������������ᷴӦ�����Ȼ��ƺ�ˮ������Һ��һ���е��������Ȼ��ƣ����NaCl��
���������⿼���˳������ʵ����ʣ���ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
�����Ŀ
�ᡢ�����������Ҫ���ʣ�
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��______��

��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽��������______�����Ͳ����������ԭ��______��______��

��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ��______���Ҵ���ˮ��Һ��______������ԡ��������ԡ����ԡ�����

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
| ����NaOH��Һ�����/mL | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.5 | 7.0 | 11.0 | 12.0 |
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ��______��������______��
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��______��

��2���۲�ͼ����С�Թ��ڵμ�ˮ�ɹ۲쵽��������______�����Ͳ����������ԭ��______��
��3����ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ��______������ԡ�?�����ԡ����ԡ���ͬ�����Ҵ���ˮ��Һ��______��

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
| ����NaOH��Һ�����/mL | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.5 | 7.0 | 11.0 | 12.0 |
�����μӵ�ָʾ������ɫʯ����Һ������������������Һ�����Ϊ13mLʱ����Һ��______ɫ��
�����μӵ�ָʾ������ɫ��̪��Һ����������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ��______��