��Ŀ����
��������������������Ƽ��ȷ����ձ�ʹ�ã�
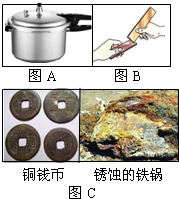
��1������ij��ҵ��Ӫ���ƶ�ͣ����һ������������е�豸�����⼣�߰ߣ�����ͬѧ���豸��һö���������˿����������ϡ�����У��۲쵽��������ʧ���÷�Ӧ�Ļ�ѧ����ʽΪ______��һ��ʱ����ֹ۲쵽�������������ݲ�����д���������ݵĻ�ѧ����ʽ______�����ֽ��ѧ���Ļ�ѧ֪ʶ�������صĹ�����Ա�����һЩ��ֹ��е�豸��һ����ʴ�Ľ��飬��д��һ�����ֵĽ��飺______��
��2���ѣ�Ti���Ǻ��캽�ա������ȷ������Ҫ�������ϣ�����Ϊ��21���͵Ľ���������ҵ����þ�ڸ�������TiCl4��Ӧ��ȡ����ѧ����ʽΪ��TiCl4+2Mg Ti+2MgCl2���÷�Ӧ˵�������ѵĻ�Ա�þ______���ǿ������������
Ti+2MgCl2���÷�Ӧ˵�������ѵĻ�Ա�þ______���ǿ������������
��3����ͭΪͭп�Ͻ���;��Ϊ�㷺��С��ͬѧ�Ӽ�������˻�ͭ��Ƭ������ͬѧ��һ�����������е�ͭ���������Ǿ����Ȳⶨ��ͭ���ᷴӦ�����������������ټ��㣮
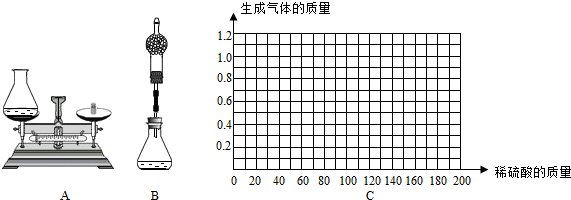
��С������Ƴ�����ͼA�����ⶨ��
������ͭ��Ʒ����ƿ��ϡ�����������
��ͭ��Ʒ������ƿ������ϡ���ᣮ
����Ӧ��Ϻ��ٲ���ƿ�ͷ�Ӧ�����������������Ӧǰ����������Ϊ����������������
��С����С�����������Ľ����飺�����������е���ƿ�ϼ�һװ�и�����ĸ���ܣ��ⶨ��Ӧǰ��װ�õ����������˵��С�����иĽ��������ǣ�______��
�ۻ���ͬѧΪ�˲ⶨ��ͭ��Ʒ��ɣ�ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���±���
| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ������g�� | 50.0 | 50.0 | 50.0 | 50.0 |
| ȡϡ����������g�� | 40.0 | 80.0 | 120.0 | 160.0 |
| ��������������g�� | 0.4 | 0.8 | 1.0 | 1.0 |
���������ڵ�1����Ʒ��õ������У�______�����������ƣ���ȫ��Ӧ�ˣ�
������ͼC�л�����50.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
���Լ����ͭм��Ʒ�е�п��������������д��������̣�����������һλС������
�⣺��1����ΪFe2O3�������ᷴӦ�����Ե���һö���������˿����������ϡ������ʱ���۲쵽��������ʧ��������������������¶���������ּ��������ᷴӦ�������������Թ۲쵽�������������ݲ���������ʽΪ��Fe2O3+6HCl�T2FeCl3+3H2O��Fe+2HCl�TFeCl2+H2������ֹ�������ж��ַ������磺��������Ʒ�������Ʒ����ˢ����ȣ�
��2�������˳�����ǰ�ߵĽ�����Ѻ�ߵĽ����û���������Ϊþ�ܰ����û�������������û��þ���ã�
��3������Ϊ�������ݳ�����ͬʱ��ˮ����Ҳ���������ݳ���������ǰ������������Ҳ����������������ˮ������������
��I��һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II��㣺��û���������ʱ����û������ķų����������ǣ�0��0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣�ÿ����40�����ᣬ����ͻ��ų�0.4�ˣ����Ҫ��ų�1.0�����壬��Ҫ���������������100�ˣ������ߵ��ǣ�100��1.0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣����������������������ı�ֵ��ȣ�б����ͬ��������ͼ����һ��ֱ�ߣ�
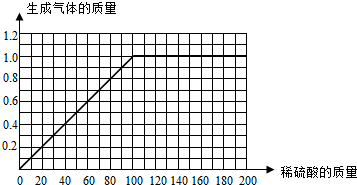
III�Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ���μӷ�Ӧ��п������Ϊx��
��μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 2
x 1.0 g

x=32.5g
���ͭм��Ʒ�е�п����������Ϊ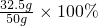 =65.0%
=65.0%
�𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
�ʴ�Ϊ��
��1��Fe2O3+6HCl�T2FeCl3+3H2O�� Fe+2HCl�TFeCl2+H2���� ��������Ʒ���������������𰸾��ɣ�
��2������
��3���ڷ�Ӧ��������ˮ�������������ݳ���
�ۢ����
��
III���𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
��������1����˿��������������Ҫ�ɷ����������������������������������ⷴӦ��������ȫ��Ӧ�����ٺ����ᷴӦ�������ݣ������������������ж�����ķ�ʽ��
��2������þ���ѵ��û���ϵ���ж����ֽ����Ļ��ǿ����
��3������������������ͬʱ���״���������ˮ���������Ӹ���ܵ�ԭ��
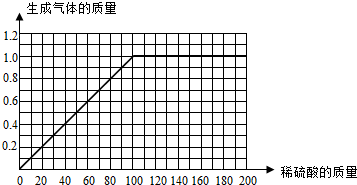
I����һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II���ҳ���㣬���ֵ�����ߵ����ƻ���ͼ�ɣ�
III���Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ�Ȼ��д����ѧ����ʽ������п��������H2SO4��������
�������������йؽ���֪ʶ�Ŀ����⣬��Ŀ���漰��֪ʶ���Լ�Ӧ�ý϶࣬�ܹ��ϺõĶ����֪ʶ����ѵ���Ϳ��飻
��2�������˳�����ǰ�ߵĽ�����Ѻ�ߵĽ����û���������Ϊþ�ܰ����û�������������û��þ���ã�
��3������Ϊ�������ݳ�����ͬʱ��ˮ����Ҳ���������ݳ���������ǰ������������Ҳ����������������ˮ������������
��I��һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II��㣺��û���������ʱ����û������ķų����������ǣ�0��0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣�ÿ����40�����ᣬ����ͻ��ų�0.4�ˣ����Ҫ��ų�1.0�����壬��Ҫ���������������100�ˣ������ߵ��ǣ�100��1.0�����Ƚϵ�һ�ݺ͵ڶ��ݿ��Է��֣����������������������ı�ֵ��ȣ�б����ͬ��������ͼ����һ��ֱ�ߣ�

III�Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ���μӷ�Ӧ��п������Ϊx��
��μӷ�Ӧ��п������Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 2
x 1.0 g

x=32.5g
���ͭм��Ʒ�е�п����������Ϊ
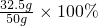 =65.0%
=65.0% �𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
�ʴ�Ϊ��
��1��Fe2O3+6HCl�T2FeCl3+3H2O�� Fe+2HCl�TFeCl2+H2���� ��������Ʒ���������������𰸾��ɣ�
��2������
��3���ڷ�Ӧ��������ˮ�������������ݳ���
�ۢ����
��

III���𣺻�ͭм��Ʒ�е�п����������Ϊ65%��
��������1����˿��������������Ҫ�ɷ����������������������������������ⷴӦ��������ȫ��Ӧ�����ٺ����ᷴӦ�������ݣ������������������ж�����ķ�ʽ��
��2������þ���ѵ��û���ϵ���ж����ֽ����Ļ��ǿ����
��3������������������ͬʱ���״���������ˮ���������Ӹ���ܵ�ԭ��
I����һ�ݺ͵ڶ��ݱȽϿ��Է��֣����������������ʱ�����������Ҳ�����ӣ�˵����һ���н���û����ȫ��Ӧ��Ҳ˵��������ȫ��Ӧ��
II���ҳ���㣬���ֵ�����ߵ����ƻ���ͼ�ɣ�
III���Ƚϵ����ݺ͵��ķݿ��Է��֣����������������ʱ��������������ֲ��䣬˵���Ͻ���ȫ��Ӧ�ų������������1�ˣ�Ȼ��д����ѧ����ʽ������п��������H2SO4��������
�������������йؽ���֪ʶ�Ŀ����⣬��Ŀ���漰��֪ʶ���Լ�Ӧ�ý϶࣬�ܹ��ϺõĶ����֪ʶ����ѵ���Ϳ��飻

��ϰ��ϵ�д�
�����Ŀ

 �������������������Ӧ��ʮ�ֹ㷺��
�������������������Ӧ��ʮ�ֹ㷺��
 �������������������Ӧ��ʮ�ֹ㷺��
�������������������Ӧ��ʮ�ֹ㷺�� �������������������Ӧ��ʮ�ֹ㷺��
�������������������Ӧ��ʮ�ֹ㷺��