��Ŀ����
 �������������������Ӧ��ʮ�ֹ㷺��
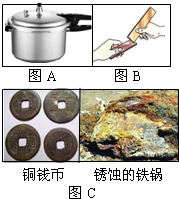
�������������������Ӧ��ʮ�ֹ㷺����1��ͼA�ǽ�����һ��Ӧ��ʵ���������˽�����
��2��ͼB���������Ƭ��̻����ڴ�ͭƬ�������ԵĻ��ۣ���ʵ��̽����Ŀ����
��3��2007��12�£����ιų������Ϻ�һ�š��ɹ����̳�ˮ��ͼC������Ʒ�����������ϡ����ϴ��ȥ��д��ϡ�������Ļ�ѧ����ʽ
��4�����㣺
��Fe2O3����Է��������ǣ�
��50t��������84%�ij����������Ͽ����ú���98%������
��������1���˽�������������ʣ������ԡ������ԡ���չ�ԡ����Եȣ�
��2���ȽϺϽ��봿ͭ��Ӳ�ȣ�
��3�������⣬������������Ӧ������ϡ���ᣮ
��4����������Է����������ڸ�Ԫ��ԭ����֮�����ڸ��ݳ��������������ĺ�����������������������ٸ�������������Ԫ�ص���������������������к�����Ԫ�ص��������������������к���Ԫ�ص�����������ʯ����Ԫ��������ȣ������������������
��2���ȽϺϽ��봿ͭ��Ӳ�ȣ�
��3�������⣬������������Ӧ������ϡ���ᣮ
��4����������Է����������ڸ�Ԫ��ԭ����֮�����ڸ��ݳ��������������ĺ�����������������������ٸ�������������Ԫ�ص���������������������к�����Ԫ�ص��������������������к���Ԫ�ص�����������ʯ����Ԫ��������ȣ������������������
����⣺��1��ͼA�ǽ�����һ��Ӧ��ʵ���������˽������������ʵĵ����ԣ�
�ʴ�Ϊ��������
��2��ͼB���������Ƭ��̻����ڴ�ͭƬ�������ԵĻ��ۣ���ʵ��̽����Ŀ���ǣ��ȽϺϽ�ʹ�������Ӳ�ȣ�
�ʴ�Ϊ���ȽϺϽ�ʹ�������Ӳ��
��3������Ʒ�����������ϡ����ϴ��ȥ��д��ϡ�������Ļ�ѧ����ʽFe2O3+6HCl=2FeCl3+3H2O
�ʴ�Ϊ��Fe2O3+6HCl=2FeCl3+3H2O
��4������Է����������ڸ�Ԫ��ԭ����֮�ͣ�Fe2O3����Է�������=56��2+16��3=160
�������к���Ԫ�ص�����������ʯ����Ԫ��������ȣ�50t��������84%�ij������У�������������=50t��84%=42t
50t�������к���Ԫ�ص�����=42t��
��100%=29.4t
�����Ͽ����ú���98%������������=
=30t
�ʴ�Ϊ160��30
�ʴ�Ϊ��������
��2��ͼB���������Ƭ��̻����ڴ�ͭƬ�������ԵĻ��ۣ���ʵ��̽����Ŀ���ǣ��ȽϺϽ�ʹ�������Ӳ�ȣ�
�ʴ�Ϊ���ȽϺϽ�ʹ�������Ӳ��
��3������Ʒ�����������ϡ����ϴ��ȥ��д��ϡ�������Ļ�ѧ����ʽFe2O3+6HCl=2FeCl3+3H2O
�ʴ�Ϊ��Fe2O3+6HCl=2FeCl3+3H2O
��4������Է����������ڸ�Ԫ��ԭ����֮�ͣ�Fe2O3����Է�������=56��2+16��3=160
�������к���Ԫ�ص�����������ʯ����Ԫ��������ȣ�50t��������84%�ij������У�������������=50t��84%=42t
50t�������к���Ԫ�ص�����=42t��
| 56��2 |
| 160 |
�����Ͽ����ú���98%������������=
| 29.4t |
| 98% |
�ʴ�Ϊ160��30
�����������ǶԽ������ϵĿ����⣬������ص����йػ�ѧʽ�ļ��㣬��Ҫ�����ˢ�������Է����������ڸ�Ԫ��ԭ����֮������Ԫ�ص������������ʵ�������Ԫ�ص����������ij˻���

��ϰ��ϵ�д�
�����Ŀ

 �������������������Ӧ��ʮ�ֹ㷺��
�������������������Ӧ��ʮ�ֹ㷺�� �������������������Ӧ��ʮ�ֹ㷺��
�������������������Ӧ��ʮ�ֹ㷺��