��Ŀ����
��9�֣�ʵ����ѧϰ��ѧ����Ҫ�ֶΣ�ͨ��ʵ���������ʵ�������ʹ��¾���
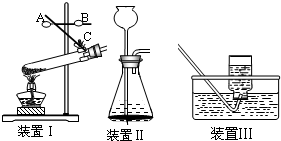
��һ��ʵ������ȡ�����dz���һ����Ҫ��ʵ�飬�������������װ��ͼ�ش��й����⣺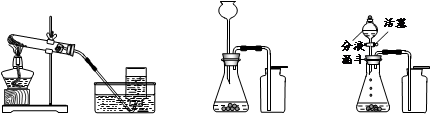
װ��I װ�â� װ�â�
��1��װ��I���ø����������������Ӧ�Ļ�ѧ����ʽΪ_________________________�����������Ѿ�������ʵ�������� ��
��2��ijͬѧ��װ�â���ȡ���ռ�������̼����ȼ�ŵ�ľ�����ڼ���ƿ��������ʼ��δ���ֻ���Ϩ��ԭ����______________________________________________��
��3����װ�â���ж������̺�˫��ˮ��ȡ������ʵ��ʱ������Ӧ���ʹ��죬���Բ�ȡ�Ĵ��������� ��
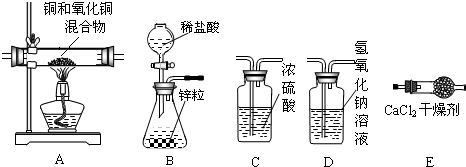
��4�������Ѿ�ѧ��ʵ������ȡO2��CO2��H2��������ķ�Ӧԭ������ȡ���ռ�������������ɳ���ȡ����������ʱ��Ӧ�Ĺ�ͬ��_______����ѡ����ţ���
| A����Ҫ���� | B����ʹ�ô��� |
| C��û������μӷ�Ӧ | D�����ɵ�����ֻ��һ�� |

��5��Ϊȷ�ⶨˮ����ɲ���ֹ���ʡ������ȸ��ţ����ܿڵ���ȷ����˳��Ϊ��
�ۡ�___��____��____��____���١��ڡ��������⡣
��6�������Aװ������Ʒ��ʵ��������6.4�ˣ�Eװ������������7.2�ˣ�Fװ��������0.1�ˣ��ݴ˿����ˮ��H��OԪ��������Ϊ��ֻд����ʽ��____________��
��7��ʵ���������A�����к�ɫ���壬���ʵ������Ӱ����________________�����������ƫ�����������ƫ������Ӱ�족����
��1��2KMnO4 K2MnO4+MnO2+O2����ƿ��������ð����
��2������©��û������Һ���£���©��û��Һ�⣬��װ��©���������Բ��ò����֣���
��3����ת��Һ©��������������Һ���µ����ʡ���4��CD����5���ߢޢܢݣ�ȫ�ԲŸ��֣���
��6��(7.2��6.4):6.4�������������Ҳ���֣���ע�⣺��0.1�أ�����7����Ӱ�졣
����

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�