��Ŀ����
ʵ����ѧϰ��ѧ����Ҫ�ֶΣ�ͨ��ʵ���������ʵ�������ʹ��¾�����һ��ʵ������ȡ�����dz�����Ҫ��һ��ʵ�飬�������ʵ��װ��ͼ�ش��й����⣺
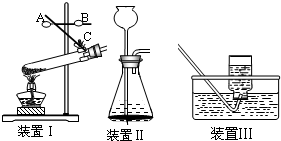
��1��װ�� I�Ǽ��ȹ����װ�ã�ָ�����еĴ���
��2��ʹ��װ�� II��ȡO2����ѧ����ʽΪ
��3��ʹ��װ�� IIǰҪ���������ԣ�������
��4��ʵ������ȡH2��O2��CO��CO2��NH3���������壬��ѡ��װ�� III���ռ���������
������ijʵ��С��Ϊ�ⶨͭ������ͭ�����������ͭ������������������ø��﴿����������ԭ����ͭ��ʵ�飬����ʵ��װ������ͼ��ʾ���ش��������⣺
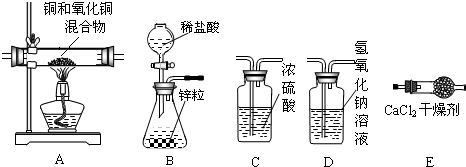
��5������ͼ���ӳ�����װ�ã�����˳������ΪB��
��6��Aװ����ʵ��ǰ���������Ϊ2.5g��ʵ����ʣ���������Ϊ2.1g���������е���������Ϊ
��7��ijͬѧ��Eװ�����ӵ���������������������ͭ����������������Ľ����
��������һ����1���������ʱ�Թ�Ӧ��������б��
��2��II�ǹ�Һ�����ķ���װ�ã��ݴ˻ش����⼴�ɣ�
��3����װ��һ���Dz���Һ�ⷨ���������Եļ��飮
��4������IIIװ���ռ�����Ҫ��֤�����岻������ˮ��
��5��ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2���������HCl�����ˮ������
��6�����÷�Ӧǰ���С��������ͭ�е�����������ͭ���������м��㣮
��7���������е�ˮ����ȫ����E���գ�����E�������˲��ֿ����е�ˮ��ʹE���ӵ��������
��2��II�ǹ�Һ�����ķ���װ�ã��ݴ˻ش����⼴�ɣ�
��3����װ��һ���Dz���Һ�ⷨ���������Եļ��飮
��4������IIIװ���ռ�����Ҫ��֤�����岻������ˮ��
��5��ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2���������HCl�����ˮ������
��6�����÷�Ӧǰ���С��������ͭ�е�����������ͭ���������м��㣮
��7���������е�ˮ����ȫ����E���գ�����E�������˲��ֿ����е�ˮ��ʹE���ӵ��������
����⣺
��1���������ʱ�Թ�Ӧ��������б����������ը���Թܣ�
��2��II�ǹ�Һ�����ķ���װ�ã���ȡ������˫��ˮ��������̵ķ�Ӧ�ǹ�Һ�����ͣ�
��3����װ��һ���Dz���Һ�ⷨ���������Եļ��飬���ж���������ͨ��©����עˮ���ۿ�Һ��ı仯��
��4������IIIװ���ռ�����Ҫ��֤�����岻������ˮ����������һ����̼����������ˮ��
��5����������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2����������װ��D����HCl��������װ��C����ˮ��������װ��E�ռ��������ܶ���С������Ϊװ��D��ʢ��������������Һ�ܺܿ�����HCl���巢����ѧ��Ӧ��
��6����Ӧǰ��������������2.5g-2.1g=0.4g��������ͭ��������
=2g������ͭ����������Ϊ
��100%=80%
��7���ڸ��������£��������е�ˮ����ȫ����E���գ�����E�������˲��ֿ����е�ˮ��ʹE���ӵ�������Ӷ�ʹ������Ľ����ʵ��ֵ��Ƚ�ƫ��
�ʴ�Ϊ����1���Թܿ�������б�����Թܿ�û����������б���� AB��
��2��2H2O2
2H2O+O2����
��3���н�װ���е��ܵ��ܣ��ӳ���©���м�����ˮ������©���д���һ��ˮ����˵���������ԽϺã�
��4��H2��O2��CO�� ��5��D��C��A�� ��6��80%�� ��7������
��1���������ʱ�Թ�Ӧ��������б����������ը���Թܣ�
��2��II�ǹ�Һ�����ķ���װ�ã���ȡ������˫��ˮ��������̵ķ�Ӧ�ǹ�Һ�����ͣ�
��3����װ��һ���Dz���Һ�ⷨ���������Եļ��飬���ж���������ͨ��©����עˮ���ۿ�Һ��ı仯��
��4������IIIװ���ռ�����Ҫ��֤�����岻������ˮ����������һ����̼����������ˮ��
��5����������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2����������װ��D����HCl��������װ��C����ˮ��������װ��E�ռ��������ܶ���С������Ϊװ��D��ʢ��������������Һ�ܺܿ�����HCl���巢����ѧ��Ӧ��
��6����Ӧǰ��������������2.5g-2.1g=0.4g��������ͭ��������
| 0.4g | ||
|
| 2g |
| 2.5g |
��7���ڸ��������£��������е�ˮ����ȫ����E���գ�����E�������˲��ֿ����е�ˮ��ʹE���ӵ�������Ӷ�ʹ������Ľ����ʵ��ֵ��Ƚ�ƫ��
�ʴ�Ϊ����1���Թܿ�������б�����Թܿ�û����������б���� AB��
��2��2H2O2
| ||
��3���н�װ���е��ܵ��ܣ��ӳ���©���м�����ˮ������©���д���һ��ˮ����˵���������ԽϺã�
��4��H2��O2��CO�� ��5��D��C��A�� ��6��80%�� ��7������
�����������ǻ�ѧʵ������������ۺ�Ӧ�ÿ����⣬����ʱҪ�Գ����ʵ������ȷ����ʶ�������ܶ��ۺ�ʵ���������������̽�֣��������ǵ��͵�ʵ��̽���⣬��������������ԭ����ͭ�����ʺ�ʵ�����ȷ�����⣬Ҫ�������ÿ��ʵ���Ŀ�ļ���ȷ�����⣮

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ