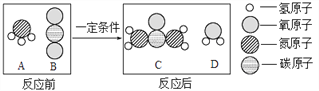
题目内容
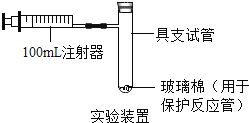
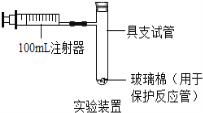
【题目】某兴趣小组对KClO3分解反应的催化剂进行研究,在相同的加热条件下,用下图装置完成表中实验:
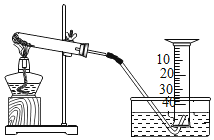
编号 | KClO3质量/g | 催化剂 | 催化剂质量/g | 收集50mLO2所需时间/s |
实验1 | 5 | - | - | 171 |
实验2 | 5 | MnO2 | 0.5 | 49 |
实验3 | 5 | Fe2O3 | 0.5 | 58 |
实验4 | 5 | KCl | 0.5 | 154 |
(1)设置实验1的目的是___________________
(2)表中所列3种催化剂的催化效果最佳的是______________
(3)写出KClO3分解的化学方程式:_________________________
(4)由实验1和实验4可知,KCl____(填“有”或“无”)催化作用。维持加热条件不变,用实验1再继续收集收集50mLO2,所需时间明显少于171s,解释原因:_____________
(5)要比较KClO3分解反应中不同催化剂的催化效果,除了测量收集50mLO2所需时间外,还可以测量相同时间内____________
【答案】对比实验 MnO2 2KClO3 2KCl+3O2↑ 有 生成的KCl加快了反应 收集气体的体积
2KCl+3O2↑ 有 生成的KCl加快了反应 收集气体的体积
【解析】
(1)实验1中没有加入可能作催化剂的物质,设置实验1的目的与其他组作实验对比,故填对比实验。
(2)收集50mLO2所需时间越短说明催化效果越佳,MnO2用时最短,所以3种催化剂的催化效果最佳的是MnO2,故填MnO2。
(3)KClO3在催化剂的催化作用和加热条件下反应生成氯化钾和氧气,故反应的化学方程式写为:2KClO3 2KCl+3O2↑。
2KCl+3O2↑。
(4)由表中数据可知,加入0.5gKCl,收集50mLO2所需时间实验1所用时间短一些,说明KCl有催化作用,故填有;
维持加热条件不变,用实验1再继续收集收集50mLO2,所需时间明显少于171s是因为氯酸钾分解生成KCl,KCl有催化作用,加快了反应,故填生成的KCl加快了反应。
(5)还可以测量相同时间内收集气体的体积来比较反应速率,故填收集气体的体积。