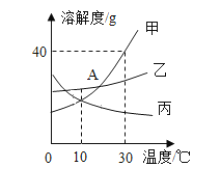
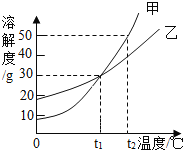
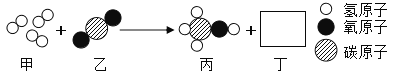
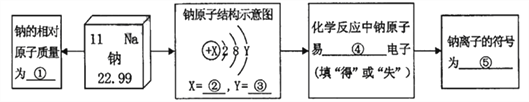
��Ŀ����
����Ŀ���Ȼ���ͭ��CuCl���㷺Ӧ����ұ�𡢵�ơ�ҽҩ����ҵ��CuCl���Ʊ��������£�
��֪����1��CuCl������ˮ���Ҵ����ڳ�ʪ�������ױ��ʡ��Ҵ���ˮ���ܣ�
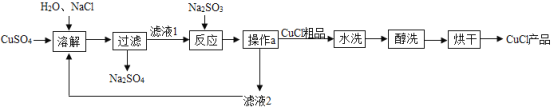
����Ӧ���з����Ļ�ѧ�仯�� 2CuCl2+Na2SO3+H2O=2CuCl��+2NaCl+H2SO4��
��1������ a ������Ϊ_____��
��2���������п���ѭ�����õ����ʣ�ˮ���⣩��_____��д��ѧʽ����
��3��CuCl��Ʒ��ˮϴ�������ˡ���ϴ��������ϴ����Ŀ����_____��
���𰸡����� NaCl ����ȥ��CuCl�����ˮ����ֹ�����
��������
��1��ͨ�������ܹ���Һ�������룬�ʲ���a�������ǹ��ˡ�
��2�����Ƿ�Ӧ�Ҳ������������ʿ���ѭ��ʹ�ã��ʱ������п���ѭ�����õ����ʣ�ˮ���⣩���Ȼ��ƣ���ϵʽΪNaCl��
��3������ϴ����Ŀ���ǿ��Գ�ȥˮ����ֹ�ں���������CuCl���ʣ��ʡ���ϴ����Ŀ���ǿ���ȥ��CuCl�����ˮ����ֹ����ʡ�

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д�����Ŀ��Ϊ�ⶨij����ͭ��ͭ�Ĺ�������������ͭ������������С��ͬѧȡ20g�������������ձ��У���100gϡ�����Ϊ�ĵȷ����μ������н���ʵ�飬����������£�
���� | �� | �� | �� | �� |
����ϡ���������/g | 25 | 25 | 25 | 25 |
ʣ����������/g | 16 | a | 10 | 10 |
�ش������⣺
��1��ԭ���������У�����ͭ����������Ϊ_____��
��2���ϱ��У�a��ֵΪ_____��ʵ����������Һ�е�������_____���ѧʽ����
��3����ʵ������ϡ���������ʵ���������Ϊ____����д��������̣������ȷ��0.1%��