��Ŀ����
����Ŀ��Ϊ�ⶨij����ͭ��ͭ�Ĺ�������������ͭ������������С��ͬѧȡ20g�������������ձ��У���100gϡ�����Ϊ�ĵȷ����μ������н���ʵ�飬����������£�
���� | �� | �� | �� | �� |
����ϡ���������/g | 25 | 25 | 25 | 25 |
ʣ����������/g | 16 | a | 10 | 10 |
�ش������⣺
��1��ԭ���������У�����ͭ����������Ϊ_____��
��2���ϱ��У�a��ֵΪ_____��ʵ����������Һ�е�������_____���ѧʽ����
��3����ʵ������ϡ���������ʵ���������Ϊ____����д��������̣������ȷ��0.1%��
���𰸡�50% 12 H2SO4��CuSO4 19.6%
��������
����ͭ��ϡ���ᷴӦ��������ͭ��ˮ��
��1���ɱ������ݿ�֪������������ͭ��ȫ��Ӧ��ʣ���10gΪͭ���ʣ���������ͭ������Ϊ10g��ԭ���������У�����ͭ����������Ϊ![]() ��100%��50%
��100%��50%
��2�����ݵ�һ�ι������4g���������κ��������䣬˵���ڶ���Ҳ����4g������a��ֵΪ12g��ʵ�������������������������Һ�е�������ʣ�����������ɵ�����ͭ����Ӧ�Ļ�ѧʽΪH2SO4��CuSO4��
��3��25gϡ������4g����ͭ��ȫ��Ӧ
���ʵ������ϡ���������ʵ���������Ϊx
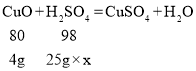
![]()
![]()
����ʵ������ϡ���������ʵ���������Ϊ19.6%��

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�