��Ŀ����
�ӻ�ѧ���ӽ���ʶ�����е��й����⣮
��1������Ӫ���ḻ���������ۡ������ʡ�ά���ء�п�������ơ����ȣ������ܸ������ṩ����������������п���ơ�������ָ�� ��������ʡ�����Ԫ�ء����ӡ�����
��2������Ʒ�������ϺƤ�Ⱥ��зḻ�ĸƣ��dz����IJ���ʳ�������ȱ�ƻᵼ���� ����
��3������ˮ����ˮ�����г��û���̿������̿����Ҫ�������� ����
��4��ϴ�Ӽ�����ϴ�;��ϵ����ۣ�������Ϊϴ�Ӽ������� �����ܣ�
��5����ͥ�����г����������У��������ޢڷϱ�ֽ ���ò�Ҷ�ܿ�Ȫˮƿ���������ڿɻ��յ�����������������ţ���
��1�����ۣ����ࣩ��������Ԫ�� ��2�����Ͳ� ��3������ ��4���黯 ��5���٢ڢ�
���������������1��ʳ���к���������Ӫ�����ʣ������ʡ����ࡢ֬����ά���ء�ˮ�����Σ�ÿһ��Ӫ�����ʶ�������������ģ��������ǹ�������ϸ���Ļ������ʣ������������������֯�ĸ��µȶ��벻�������ʣ����⣬�����ʻ��ܱ��ֽ⣬Ϊ�����������ṩ��������������������Ҫ�Ĺ������ʣ������һ�л������ѧϰ����·�������ͺ����������ĵ�������Ҫ�������࣬����Ҳ�ǹ���ϸ����һ�ֳɷ֣�ˮ��ά���غ����β���Ϊ�����ṩ������п���ơ����Ƕ�����п���ơ���ԭ�ӵ��ܳƣ��⼴��Ԫ�أ�������ԭ�ӣ�Ҳ���ǵ��ʣ���ָ����Ԫ�أ���2�������к��еĸơ����ǹ��ɹ��������ݵ���Ҫ�ɷ֣�����ȱ�ƻỼ���Ͳ����������˺�Ůȱ���ù������ɣ���Ϊ�������³´�л�仺���������������������еĸ���ʧ���������������֢����3������̿�����������ã��ܳ�ȥˮ��ɫ�غ���ζ�����ʣ���4����ϴ�·��ϵ����ۣ�ԭ������ϴ�Ӽ��������£������Լ�С��Һ�ξ�����������һ��Һ�����γ�����Һ�Ӷ���ȥ�����е��黯����������þͽ��黯���ã���5�����������ڽ������ϱ�ֽ���ڿɻ������Ȫˮƿ���ڿɻ�����ò�Ҷ���ڲ��ɻ����
���㣺�����Ԫ�������Ԫ�ض����彡������Ҫ���ã�����ˮ�����������뾻���������黯�������黯���ã������������Ӫ���أ�������Ⱦ�����Դ��Σ����������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�������Ի�ѧ��
��1�������е�ȼ��
���Ż��Ƚϣ�����ú Сľ�������������������=��������ú¯�����У������������Ŀ���� ��ú����ȫȼ�ղ���һ���ж�����Ļ�ѧʽ�� ��
����ˮ����ԭ���� ������ˮ��Ĥ��ĭ���������������ʱ��������Һ�����ĭ����������չ�γ�һ��ˮĤ���������ԭ���� ��
��2����������������;�㷺
������������Ʒ����Ҫ���ɽ��������Ƴɵ��� ��
�����������ʳ��ʱ������������м��θ����θ�����Ҫ�ɷ�Ϊ���ᣩת��Ϊ�ɱ����յ�Fe2+����Ӧ�Ļ�ѧ����ʽΪ ��2�֣����� ��Ӧ���������Ӧ���ͣ���
����������װҩ���Ҫ���������� �ԣ�
��ͭ���кܺõ� �ԣ��ʿ������ߡ�����ʪ��ұ���������ָ ���ѧ����ʽ2�֣���ͭ�Ϳ����е�O2��H2O��������ͭ���Cu2(OH)2CO3�ݣ��жϵ������� ��
��3�������е�ˮ����Һ
�ٴ�����Ȼˮʱ�����õĻ������� �������ƣ�������������һ�����͵����������仯ѧʽΪ ��
��������500mL 0.9%��������ˮ���ܶ�Ϊ1.0 g/mL������ҪNaCl������Ϊ g�����ƹ����У��������������� ������NaClʱ����������������̣�1 g���������룩��������������ȷ����������Һ���������������� 0.9%�����������������=����
����ͼ�Ǿ�����ˮ�ļ���װ�ã�����˵����ȷ���� ��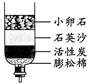
| A���������ˮ�Ǵ����� | B����װ���ܶ�ˮɱ������ |
| C����װ���ܰ�Ӳˮ��Ϊ��ˮ | D������̿������ˮ�е�ɫ�ؼ���ζ |
�������ز�������ź������հ�װ���ӳ���ʳƷ�ı����ڣ�ԭ���� ��
�ڽ�N2�����װ������������������ΪN2�Ļ�ѧ���� ������á����ȶ�������
����ͼ�ǡ�504˫�������ı�ǩ�����ʴ��������⣺

a���������ٺ��� �ֵ��ʣ�
b��Ϊ�ⶨ��˫������ʹ��Ч����ȡ����˫����������ͼ��ʾ��ʵ�飬һ��ʱ������Թ���ˮ����� 21%�����������������=������������ ��
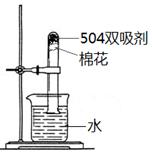
c������ʧЧ��˫�����У��к���ɫ���壬����Ҫ�� ��
d����˫�����У�NaCl�����ÿ����� ��
�±���100gijʳƷ�IJ���Ӫ���ɷ֣������ɷֲ����ṩ��������
| ������ | ������ | ���� | ��֬ | ˮ | �� | �� | �� | |
| 2060kJ | 50g | 20g | ���� | 5g | 4.6mg | 3.3mg | 8mg | |
��1����ʳƷ�������л�Ӫ������ �����ʡ����ࡢ��֬ ��ÿ100g��ʳƷ������Ӫ���ɷ����������� ��
��2���ϱ����ơ��ס���ָ���� ��ѡ����ʡ�����Ԫ�ء��������ӡ�����
��3������ʳƷ�еĸ���̼��Ƶ���ʽ���ڣ���100g��ʳƷ�У�̼���Ϊ mg��
��4������ʳ������������ɵ����ʡ����ࡢ��֬�ṩ�ģ���λ�����ĵ����ʡ����ࡢ��֬��ȫ��������ԼΪ����֬ 38kJ/g�������� 20kJ/g������ 15kJ/g��
���ݴ����ݼ��㣬������100g��ʳƷ����֬������Ϊ g��
ij����Ʒ��Ӫ���ɷּ���
| ��Ŀ | ÿ100mL | NRV% |
| ���� | 290kJ | 3% |
| ������ | 3.2g | 5% |
| ֬�� | 4.0g | 7% |
| ̼ˮ������ | 4.8g | 2% |
| ���� | 62mg | 3% |
��1�� ��ˮҲ���������Ļ���Ӫ�����ʣ����ڱ���δ��ʾ��
��2��������Ʒ�� �ܹ��ṩ��������дһ�֣���
��3������Ʒ�к��зḻ�ĸ� ������ʡ�����ԭ�ӡ���Ԫ�ء�������ͯÿ��Ҫ��ȡ�㹻���ĸƣ����ܱ��� ������ĸ��ţ���
a��ƶѪ֢ b�����Ͳ� c����״���״�
��4����ͼ������Ʒ�����ְ�װ��������ʹ�õ���Ҫ���������л��ϳɲ��ϵ��� ������ĸ��ţ���ͬ�����������ϳɲ��ϵ��� ��Ӧ�������
 ����������� ��
����������� ��
��5���������ʲ���ʹ������ʧȥԭ���������Ե��� ������ĸ��ţ���
a��Ũ���� b������Ǧ c������� d����ȩ




