��Ŀ����
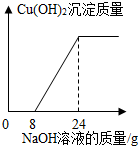
ij����С���һ�����ŷŵķ�ˮ�к���H2SO4��CuSO4��Ϊ�˲ⶨ��ˮ��CuSO4��������������С��ȡ��100g��ˮ����μ���10%��NaOH��Һ���������������Cu��OH��2��������������NaOH��Һ������ϵ����ͼ��ʾ��
��1����H2SO4��Ӧ��NaOH��Һ����Ϊ______g��100g��ˮ��H2SO4������Ϊ______g��
��2�������ˮ��CuSO4������������
��1����H2SO4��Ӧ��NaOH��Һ����Ϊ______g��100g��ˮ��H2SO4������Ϊ______g��
��2�������ˮ��CuSO4������������

��1���ɹ�ϵͼ��֪����8gNaOH��Һ���ˮ�е����ᷢ����Ӧ��
���ˮ����������Ϊx
2NaOH+H2SO4=Na2SO4+2H2O
80 98
8g��10% x
80��98=��8g��10%����x
��֮�� x=0.98g
�ʴ�Ϊ��8��0.98��
��2���ɹ�ϵͼ��֪�����ˮ������ͭ��Ӧ��NaOH��Һ������=24g-8g=16g������NaOH������=16g��10%=1.6g
���ˮ������ͭ������Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
16080
y1.6g
160��80=y��1.6g
��֮�� y=3.2g
��ˮ��CuSO4����������=
��100%=3.2%
�𣺷�ˮ��CuSO4����������Ϊ3.2%��
���ˮ����������Ϊx
2NaOH+H2SO4=Na2SO4+2H2O
80 98
8g��10% x
80��98=��8g��10%����x
��֮�� x=0.98g
�ʴ�Ϊ��8��0.98��
��2���ɹ�ϵͼ��֪�����ˮ������ͭ��Ӧ��NaOH��Һ������=24g-8g=16g������NaOH������=16g��10%=1.6g
���ˮ������ͭ������Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
16080
y1.6g
160��80=y��1.6g
��֮�� y=3.2g
��ˮ��CuSO4����������=
| 3.2g |
| 100g |
�𣺷�ˮ��CuSO4����������Ϊ3.2%��

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�
�����Ŀ
 ��������һλС����
��������һλС����