��Ŀ����
����Ŀ��ʵ����ʹ�ø��������ȡ���������ղ���������̣��ɹ�ѡ����������£�
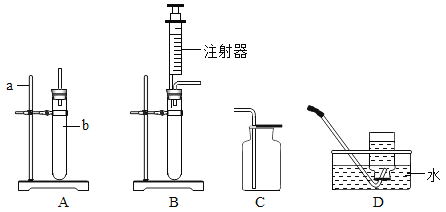
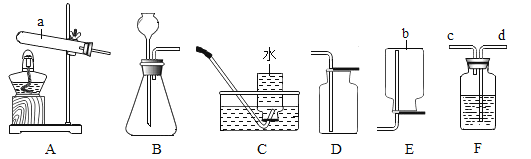
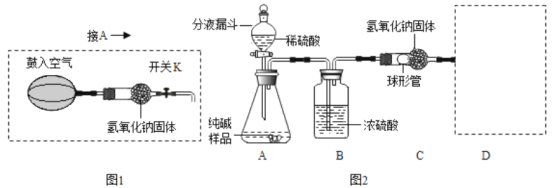
��1����ͼ��ʾ�����٢ڵ����ƣ���_______________����_________________��
��2����ȡ�����ķ���ʽΪ_____________��
��3��ѡ����C�ռ��������ŵ���_____����ѡ����D�ռ�����Ӧ��____�˽���������a������b�� ����
��4������Bװ�ý����յĶ�������������������������װ�õ��ŵ���______������ţ���
A�����ڿ��Ʒ�Ӧ���� B����������Һ��ҩƷ C��������������
��5��ʵ��������Bװ��Ҳ���Ʊ�CO2������ʽΪ____________________��
���𰸡���ƿ ����ƿ ![]() �����ϴ��� a AB CaCO3 +2HCl = CaCl2+ CO2�� + H2O
�����ϴ��� a AB CaCO3 +2HCl = CaCl2+ CO2�� + H2O
��������
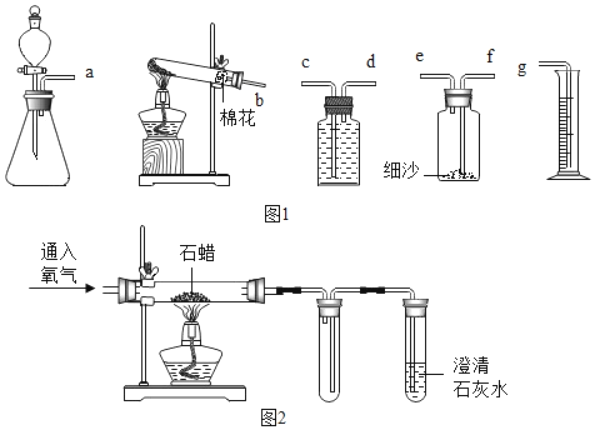
��1��������ƿ�����Ǽ���ƿ��
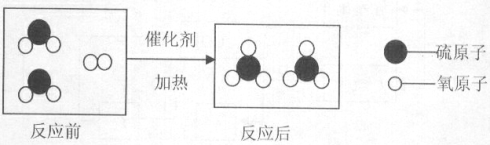
��2������������ȷֽ���������ء��������̺���������Ӧ�ķ���ʽΪ��
![]() ��
��
��3��ѡ����C�ռ��������ŵ����ռ�����������������ѡ����D�ռ����������������ܶȱȿ�����Ӧ��a�˽�����
��4������Bװ�ý����յĶ�������������������������װ�õ��ŵ��ǣ�ͨ����Һ©���ܹ�����Һ��ҩƷ�������Ӷ����ڿ��Ʒ�Ӧ������ͨ����Һ©����������Һ��ҩƷ��
��5��ʵ��������Bװ��Ҳ���Ʊ�CO2������ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O��