��Ŀ����
��2011?���ݣ�Ϊ�˼��������Σ�����ҹ��з��ˡ���ˮ�����������ա����乤�����̴����ǣ�
I���Ӹߴ������µĺ�ˮ��pH=8.1-8.3��ϴ�Ѵ��¶���ȼú�����е�SO2����SO2+H2O=H2SO3H2SO3�����ᣩ����ˮ��Ϊ�����ԣ�
����ϴ��������ĺ�ˮ��������ֽӴ����������з�Ӧ��
4NaC1+O2ʮ2H2SO3�T2Na2SO4+4HC1
III���ٽ���������ֽӴ��ĺ�ˮ��������Ȼ��ˮ��ϵȴ�ʩ��ʹ����ָ��ӽ���Ȼ��ˮ�����ŷţ�
��1����������ͼ�л�������ˮ�����������ա�ȫ�����к�ˮ��pH���α仯�������ƣ�
��2����������������Ȼ��ˮ�Ӵ��������Ϊ�����ԣ���˵���ڴ˹����к�ˮ�е�
��3������Ҫ�ⶨ��ˮ��Na2SO4������ʵ����������ǣ�
ȡ�������⺣ˮ���ⶨ
I���Ӹߴ������µĺ�ˮ��pH=8.1-8.3��ϴ�Ѵ��¶���ȼú�����е�SO2����SO2+H2O=H2SO3H2SO3�����ᣩ����ˮ��Ϊ�����ԣ�
����ϴ��������ĺ�ˮ��������ֽӴ����������з�Ӧ��
4NaC1+O2ʮ2H2SO3�T2Na2SO4+4HC1
III���ٽ���������ֽӴ��ĺ�ˮ��������Ȼ��ˮ��ϵȴ�ʩ��ʹ����ָ��ӽ���Ȼ��ˮ�����ŷţ�
��1����������ͼ�л�������ˮ�����������ա�ȫ�����к�ˮ��pH���α仯�������ƣ�
��2����������������Ȼ��ˮ�Ӵ��������Ϊ�����ԣ���˵���ڴ˹����к�ˮ�е�
����������
����������
���Ӻ�H2SO3 ������������ӷ����˷�Ӧ����3������Ҫ�ⶨ��ˮ��Na2SO4������ʵ����������ǣ�
ȡ�������⺣ˮ���ⶨ
���⺣ˮ����
���⺣ˮ����
������������BaCl2
BaCl2
��Һ��д��ѧʽ�����ټ���ϡ���ᡢ���ˡ�ϴ�ӡ���ɡ�����������������������1�������������PHֵ�Ĺ�ϵ���DZ��⣻��2�����ݴӸߴ������µĺ�ˮ��pH=8.1-8.3��˵�������������ӣ���3����������������ñ����ӣ�
����⣺��1���ɺ�ˮ��Ϊ�����Լ�PHֵԽ��ԽС���ٽ��ȫ�����к�ˮ��pH���α仯��PHֵ�ֿ�ʼ��Ϳ��������𰸣�
��2����ˮ��pH=8.1-8.3��˵���Լ��ԣ����������������ӣ�
��3���ⶨ��ˮ��Na2SO4������������Ҫ֪����ˮ���������ٸ������Ȼ�����Ӧ���ɳ�����������������Ƶ�������
�ʴ�Ϊ����1��
��2������������
��3�����⺣ˮ������BaCl2��
��2����ˮ��pH=8.1-8.3��˵���Լ��ԣ����������������ӣ�
��3���ⶨ��ˮ��Na2SO4������������Ҫ֪����ˮ���������ٸ������Ȼ�����Ӧ���ɳ�����������������Ƶ�������
�ʴ�Ϊ����1��

��2������������
��3�����⺣ˮ������BaCl2��
������ͨ���ش���֪����PHֵ���������Ĺ�ϵ���˽��˲ⶨ���������Ȼ�����ʵ��Ĺ��̣�

��ϰ��ϵ�д�
�����Ŀ
��2011?���ݣ�ijѧУ��ѧ��ȤС�������һ��ʵ�飬��ģ���о�CO��Ũ�������Ƿ������������ЧӦ�������Dz������й����ݣ�
������������ʵ��Ͳ������裺
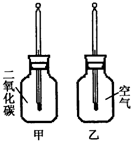
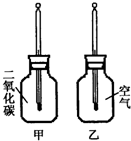
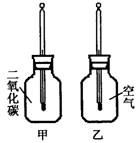
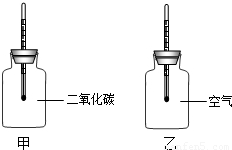
������ֻͬ���IJ���ƿ��ֱ����CO2�Ϳ����������Ϊ�ס��ң���������ͬ���¶ȼƵ���Ƥ�����ٰ���ֻ����ƿ�������� �����䣨����ͼ�����۲�ס���ƿ�е��¶ȱ仯��
������������䣬���һ��ʱ�����������ƿ�¶�ֵ������¼
�����±���
��ش��������⣺
��1��д��ʵ������ȡCO2�Ļ�ѧ����ʽ______��
��2����ƿ�г�CO2ʱ����֤ƿ���ѳ�����CO2�ķ�����______��
��3����ʵ���У�����ͬ��ʱ�䣬�����ϱ������ݣ��Ƚϼס���ƿ�¶ȱ仯�Ĺ�����______��
��4����ʵ���У������������£�Ӱ��ס���ƿ�¶Ȳ�ͬ��ԭ����CO2������ЧӦ���⣬��
�еĿ���ԭ���ǣ�д��һ�㼴�ɣ�______
��5����ͬѧ��Ϊ���ݸ�ģ��ʵ���Ŀ�ģ�ʵ����ƴ������⣬����Ϊ�ǣ�д��һ�㼴�ɣ�______��
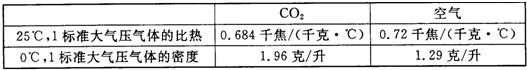
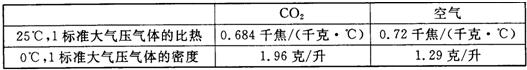
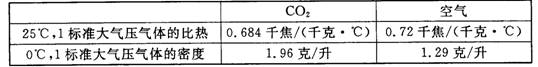
| CO2 | ���� | |
| 25�棬1������ѹ����ı��� | 0.684ǧ��/��ǧ��?�棩 | 0.72ǧ��/��ǧ��?�棩 |
| 0�棬1������ѹ������ܶ� | 1.96��/�� | 1.29��/�� |
������ֻͬ���IJ���ƿ��ֱ����CO2�Ϳ����������Ϊ�ס��ң���������ͬ���¶ȼƵ���Ƥ�����ٰ���ֻ����ƿ�������� �����䣨����ͼ�����۲�ס���ƿ�е��¶ȱ仯��

������������䣬���һ��ʱ�����������ƿ�¶�ֵ������¼
�����±���
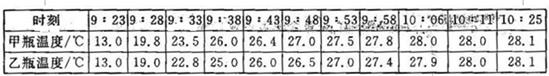
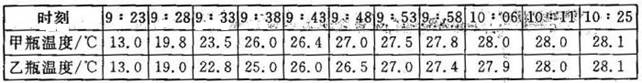
| ʱ�� | 9��23 | 9��28 | 9��33 | 9��38 | 9��43 | 9��48 | 9��53 | 9��58 | 10��06 | 10��11 | 10��25 |
| ��ƿ�¶�/�� | 13.0 | 19.8 | 23.5 | 26.0 | 26.4 | 27.0 | 27.5 | 27.8 | 28.0 | 28.0 | 28.1 |
| ��ƿ�¶�/�� | 13.0 | 19.0 | 22.8 | 25.0 | 26.0 | 26.5 | 27.0 | 27.4 | 27.9 | 28.0 | 28.1 |
��1��д��ʵ������ȡCO2�Ļ�ѧ����ʽ______��
��2����ƿ�г�CO2ʱ����֤ƿ���ѳ�����CO2�ķ�����______��
��3����ʵ���У�����ͬ��ʱ�䣬�����ϱ������ݣ��Ƚϼס���ƿ�¶ȱ仯�Ĺ�����______��
��4����ʵ���У������������£�Ӱ��ס���ƿ�¶Ȳ�ͬ��ԭ����CO2������ЧӦ���⣬��
�еĿ���ԭ���ǣ�д��һ�㼴�ɣ�______
��5����ͬѧ��Ϊ���ݸ�ģ��ʵ���Ŀ�ģ�ʵ����ƴ������⣬����Ϊ�ǣ�д��һ�㼴�ɣ�______��