��Ŀ����
����Ŀ�������Ũ�Ȳ�ͬ����ѧ���ʻ��кܴ���졣��65 gп���뵽150 g��������Ϊ98%��Ũ�����У�����п���ܽ⣬�����д̼�����ζ�����壬���������γ��������Ҫ���塣���ŷ�Ӧ���Ͻ��У�����Ũ�ȱ�С�����ϡ���ᣬ��ʱ����������û�д̼�����ζ������п������ǡ����ȫ��Ӧ��ʣ�����Һֻ����һ�����ʡ�
(1)��д��п��ϡ���ᷴӦ�Ļ�ѧ����ʽ_______��п��Ũ���ᷴӦ�Ļ�ѧ����ʽ_______��
(2)���ɵ�������������________g��
���𰸡� Zn��H2SO4 (ϡ)=== ZnSO4��H2�� Zn��2H2SO4(Ũ)=== ZnSO4��SO2����2H2O 33
��������(1)���ݸ�������Ϣ��п��Ũ�������ɶ�������ϡ��������������п��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ Zn+H2SO4=ZnSO4+H2����п��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Zn+2H2SO4��Ũ��=ZnSO4+2H2O+SO2����(2)150g��������Ϊ98%��Ũ�����к����������Ϊ150g��98%=147g������Ũ���ᷴӦ��п������Ϊx�����ĵ����������Ϊy��
Zn+2H2SO4��Ũ��=ZnSO4+2H2O+SO2��
65 196
x y
![]()
y=![]()
Zn+H2SO4=ZnSO4+H2��
65 98
65g-x 147g-y
![]()
�������Ͽɵ�x=32.5g
��п��һ���Ũ���ᷴӦ��һ���ϡ���ᷴӦ��
�����ɵĶ������������Ϊa�����ɵ�����������Ϊb��
Zn+2H2SO4��Ũ��=ZnSO4+2H2O+SO2��
65 64
32.5g a
![]()
a=32g
Zn+H2SO4=ZnSO4+H2��
65 2
32.5g b
![]()
b=1g
���ɵ����������Ϊ32g+1g=33g��

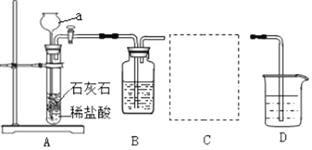
����Ŀ������С��ͬѧ����ͼA��ͼB��ʾװ����֤̼���Ƶ����ʡ�

��1������ͼAװ�ý�������ʵ��
����һ��������ƿ�ڵ�̼������Һ�еμӷ�̪����Һ�Ժ�ɫ��
���������ע����1����ͷ��ƿ���ƽ���ʹ��ͷ�¶˽���ƿ��Һ���£�������ע��������ʱע����1�з�Ӧ�Ļ�ѧ����ʽΪ �����������������ͷ��������ͼʾ��ʾλ�á�
����������֤̼������̼���Σ�Ӧ�ý��е�ʵ������� ��������ʵ�������� ��
��2������ͼBװ�ý���ʵ�顣��������̼����ͨ�뱥��̼������Һ�У�������ӦΪ��Na2CO3 + CO2 + H2O = 2NaHCO3�����������ǵ�ԭ���dz���Ӧ����ˮʹ�ܼ��������⣬���е�����ԭ���� ���������±���Ϣ�ش𣩡�
���� | 20��ʱ�ܽ��/g | ��Է������� |
Na2CO3 | 21.5 | 106 |
NaHCO3 | 9.6 | 84 |
����Ŀ����ȡ����Ķ�����̼���壬����֤������̼����������������Һ���Է�����ѧ��Ӧ���������̽��ʵ�顣
���������ϡ�
(1)̼���ơ�ˮ��������̼���Ի�������̼�����ơ�
(2)20�棬�������ơ�̼���ƺ�̼�����Ƶ��ܽ�����±���ʾ
���� | NaOH | Na2CO3 | NaHCO3 |
�ܽ��/g | 110 | 21.8 | 9.6 |
���ⶨ��ʵ�������װ��D���¶ȱ���20����
������̽����
(1)����a��������____________________��
(2)װ��B��ʢ�� NaHCO3��Һ��Ŀ��������װ��A�лӷ�����HCl���壬д��B�з�����Ӧ�Ļ�ѧ����ʽ��___________��
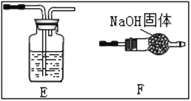
(3)װ��C��������________��ѡ��________(ѡ��E��F)�ﵽʵ��Ŀ�ģ�
(4)��ʵ�������װ��D�й۲쵽__________��˵��CO2��NaOH��Һ�ܷ�����Ӧ��
���������㡿
(5)װ��D��ʢ�е�210g20��NaOH������Һ�к���NaOH___________g��
(6)װ��D��NaOHȫ��ת����Na2CO3ʱ������������������Na2CO3_______________��?
(7)����װ��D��ͨ��������CO2ʱ�����Խ�NaOHȫ��ת��ΪNaHCO3��ʵ������л������� NaHCO3����________g(�����ǽᾧˮ)��