��Ŀ����
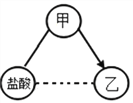
����Ŀ��ͼ1װ����ܴ���ȼ�ճ���ʢ��������п�����ҹ�ʢ��ϡ���ᡣ
(1)�ر�K3����K1��K2�������ҹ�Һ���½���������ֵ�������_______________________��
д������������Ļ�ѧ����ʽ_______________________��
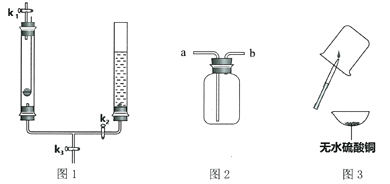
(2)��K1�ĵ��ܿڴ�����Ƥ������ͼ2��_________________(����a������b��)���ռ����塣
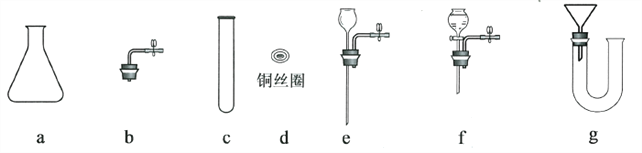
(3)��ͼ3����ʵ�飬����Ƥ����K1�����Ӳ���ȼ����ܣ��ɹ۲쵽��������________________��
(4)Ϊ��ʹ��Ӧֹͣ����ȷ�IJ�����____________________����װ�õ��ŵ���____________________��
(5)�����в�����������װһ��������װ������ͬ�ŵ��װ�ã��ñ�ű�ʾ��װ��װ��__________��
���𰸡� ���Һ����������Һ�Ӵ����������� Zn + H2SO4 �� ZnSO4 + H2�� b ��������ɫ���棬�ų��������ձ��ڱ���ˮ�飬�������й����ɰ�ɫ����ɫ �ر�K1 �濪���ã������ͣ cde��bdg
��������(1)�ر�K3����K1��K2���ҹ�Һ���½������Һ����������Һ�Ӵ����������ݣ��������ݵ�ԭ����ϡ������п��Ӧ��������п����������Ӧ����ʽΪZn + H2SO4=ZnSO4 +H2����
��2��ͼ2�Ƕ��װ�ã������������ܶȱȿ������ܶ�С��ѡ�����ſ������ռ�����Ӧ�ô�b�ܽ��룻
��3������ȼ�ղ�������ɫ���棬�ų��������ձ��ڱ���ˮ�飬�������й����ɰ�ɫ����ɫ��
��4�����ر�K1���������ѹ���ӣ�Һ�屻ѹ���ҹܣ���Һ���룬��Ӧֹͣ����װ�õ��ŵ��DZ��ڿ��Ʒ�Ӧ�ķ�����ֹͣ���濪���ã������ͣ��
��5���µ�װ��Ҫ�и��彫������ڸ����ϣ�ͼ��d���Դ�����壬��ѡcde��bdg��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���±���KNO3��NaCl�ڲ�ͬ�¶��µ��ܽ������λ��g/100g ˮ����
�¶������� | 0 | 20 | 40 | 60 | 80 | 100 |
KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 | 246 |
NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 |
��20��ʱ�ܽ�Ƚϴ��������_________ ��
�ڳ�ȥKNO3�����л��е����� NaCl��ͨ���ܽ�������Ũ����______��������ϴ��������õ��Ƚϴ�����KNO3���塣��Һ�����ʵijɷ���___________��
���� 4 ֻС�ձ�ȡ T��ʱ��ˮ�� 50 ��������ʵ�鲢��¼�������£�
ʵ���� | 1 | 2 | 3 | 4 |
KNO3 ����/g | 40 | 50 | 60 | 70 |
H2O����/g | 50 | 50 | 50 | 50 |
��Һ����/g | 90 | 100 | 105 | X |
��.ʵ�� 1 ������Һ����������Ϊ_______ ��ʵ�� 4 �� X ��ֵΪ _________��
��. T��ʱKNO3 ���ܽ���� _________ g/100g ˮ��