��Ŀ����
һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2��Ϊ�˲ⶨ����Ʒ��CaCO3�������������ֽ�100g����ȷ�5�μӵ�35g����Ʒ�У��õ�����������ͼ��
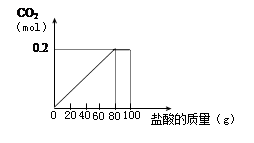
��1��ʯ��ʯ��Ʒ��CaCO3������������ ��
��2����3�μ��������aΪ g��
��3����Ӧ������0.2mol CO2������������ʵ����������������ݻ�ѧ����ʽ��ʽ���㣩

| ���� | ��1�� | ��3�� | ��4�� |
| �������������(g) | 20 | 20 | 20 |
| ʣ����������(g) | 30 | a | 15 |
��1��ʯ��ʯ��Ʒ��CaCO3������������ ��
��2����3�μ��������aΪ g��
��3����Ӧ������0.2mol CO2������������ʵ����������������ݻ�ѧ����ʽ��ʽ���㣩
��1��0.571��57.1%��4/7��
��2��20
��3��0.1825
��2��20
��3��0.1825
����������ɱ������֪����ʣ��Ĺ��弴Ϊ��������Ϊ15g����Ϊ����������ϡ�����Ӧ������̼���Ϊ20g����������Ϊ20��35����4/7��ÿ�μӵ������������һ���ģ��������ĵ�̼���ҲӦ������ͬ�ģ���ÿ�η�Ӧ��5g��̼��ƣ�һ����20g̼��ƣ����Ե����ι���Ӧ��̼���15g��ʣ���������Ϊ20g��aΪ20g�����ݻ�ѧ����ʽ���м��㣺
����Ҫ������HClx mol
CaCO3+2HCl��CaCl2+CO2��+ H2O
2 1
X 0.2
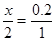
x="0.4mol"
0.4��36.5g/mol="14.6g"
14.6/80=0.1825

��ϰ��ϵ�д�
�����Ŀ

 C6H12O7+Cu2O��+2H2O
C6H12O7+Cu2O��+2H2O