ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΜ·―ßΨΆ‘ΎΈ“Ο«…μ±ΏΘ§«κ”ΟΥυ―ßΜ·―ß÷Σ ΕΜΊ¥πœ¬Ν–Έ ΧβΓΘ
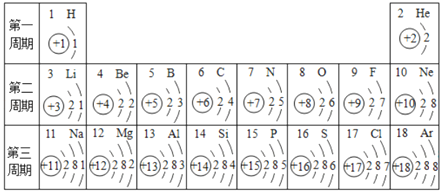
Θ®1Θ©ΫπΗ’ ·ΚΆ ·ΡΪΕΦ «”…ΧΦ‘ΣΥΊΉι≥…ΒΡΒΞ÷ Θ§ΒΪ «”…”Ύ_____Θ§“ρ¥ΥΥϋΟ«ΒΡΈοάμ–‘÷ ¥φ‘ΎΉ≈Ϋœ¥σ≤ν“λΘΜ
Θ®2Θ©”Ο“Μ―θΜ·ΧΦΚΆ¥≈ΧζΩσ ·Θ®÷ς“Σ≥…Ζ÷ «Fe3O4Θ©ΝΕΧζΘ§ΤδΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «_____ΓΘ
Θ®3Θ©¬Ν÷ΤΤΖ–Έ≥…÷¬Οή±ΘΜΛΡΛΒΡΜ·―ßΖΫ≥Χ Ϋ «_____ΓΘ
Θ®4Θ©–ΓΟτΕ‘¬η¬ηΥΒΘΚΓΑΡψΥΒ≤Λ≤Υ÷–ΗΜΚ§ΧζΘ§ΈΣ ≤Ο¥Έ“”Ο¥≈ΧζΈόΖ®Α―≤Λ≤ΥΈϋΤπά¥ΡΊΘΩΓ±–ΓΟτ»œΈΣΒΡΧζ «÷Η_____ΓΘΘ®―ΓΧνΓΑΧζ‘ΣΥΊΓ±ΜρΓΑΧζΒΞ÷ Γ±Θ©ΓΘ
ΓΨ¥πΑΗΓΩΧΦ‘≠Ή”≈≈Ν–ΖΫ Ϋ≤ΜΆ§ Fe3O4+4CO![]() 3Fe+4CO2 4Al+3O2®T2Al2O3 ΧζΒΞ÷
3Fe+4CO2 4Al+3O2®T2Al2O3 ΧζΒΞ÷
ΓΨΫβΈωΓΩ
Θ®1Θ©ΨωΕ®Έο÷ –‘÷ ΒΡ «Έο÷ ΒΡΉι≥…ΚΆΫαΙΙΘ§ΫπΗ’ ·ΚΆ ·ΡΪΕΦ «”…ΧΦ‘ΣΥΊΉι≥…ΒΡΒΞ÷ Θ§ΒΪ «”…”ΎΧΦ‘≠Ή”≈≈Ν–ΖΫ Ϋ≤ΜΆ§Θ§“ρ¥ΥΥϋΟ«ΒΡΈοάμ–‘÷ ¥φ‘ΎΉ≈Ϋœ¥σ≤ν“λΘΜ
Θ®2Θ©ΗΏΈ¬ΧθΦΰœ¬Θ§ΥΡ―θΜ·»ΐΧζΚΆ“Μ―θΜ·ΧΦΖ¥”Π…ζ≥…ΧζΚΆΕΰ―θΜ·ΧΦΘ§ΤδΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «ΘΚFe3O4+4CO![]() 3Fe+4CO2ΘΜ
3Fe+4CO2ΘΜ
Θ®3Θ©¬Ν÷ΤΤΖ–Έ≥…÷¬Οή±ΘΜΛΡΛΘ§ «“ρΈΣ≥ΘΈ¬œ¬Θ§¬ΝΡήΚΆ―θΤχΖ¥”Π…ζ≥…÷¬ΟήΒΡ―θΜ·¬Ν±ΘΜΛΡΛΘ§ΡήΉη÷Ι¬ΝΒΡΫχ“Μ≤Ϋ–β ¥Θ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ4Al+3O2®T2Al2O3ΘΜ
Θ®4Θ©–ΓΟτ»œΈΣΒΡΧζ «÷ΗΧζΒΞ÷ Θ§’β «“ρΈΣΧζΒΞ÷ Ρή±Μ¥≈ΧζΈϋ“ΐΓΘ

ΓΨΧβΡΩΓΩΈΣΝΥΦλ≤β ·Μ“ ·―υΤΖ÷–ΧΦΥαΗΤΒΡΚ§ΝΩΘ§ΦΉΓΔ““ΓΔ±ϊΓΔΕΓΥΡΈΜΆ§―ßΖ÷±π”Ο÷ ΝΩΖ÷ ΐœύΆ§ΒΡ―ΈΥα”κ―υΤΖ≥δΖ÷Ζ¥”ΠΫχ–– Β―ι≤βΕ®ΓΘ“―÷ΣΘ§―υΤΖ÷–ΒΡ‘”÷ ≤Μ»ή”ΎΥ°Θ§«“≤Μ”κ―ΈΥαΖ¥”ΠΓΘ≤βΒΟ ΐΨί»γœ¬±μΘΚ
ΦΉ | ““ | ±ϊ | ΕΓ | |
Υυ»Γ ·Μ“ ·―υΤΖ÷ ΝΩΘ·g | 10.0 | 10.0 | 10.0 | 10.0 |
Φ”»κ―ΈΥαΒΡ÷ ΝΩΘ·g | 20.0 | 30.0 | 45.0 | 50.0 |
Θ”ύΙΧΧεΒΡ÷ ΝΩΘ·g | 6.0 | 4.0 | 2.0 | 2.0 |
«κΜΊ¥πΘΚ
Δ≈ ―υΤΖ÷–ΧΦΥαΗΤΒΡ÷ ΝΩΖ÷ ΐ «_______ΘΜ
ΔΤ10.0 g―υΤΖ”κ45.0 g―ΈΥα≥δΖ÷Ζ¥”ΠΚσΘ§―ΈΥα «ΖώΜΙ”– Θ”ύ_______(ΧνΓΑ «Γ±ΜρΓΑΖώΓ±)ΘΜ
Δ«10.0 g―υΤΖ”κΉψΝΩœΓ―ΈΥαΖ¥”ΠΚσΩ…≤ζ…ζΕΰ―θΜ·ΧΦ_____ΩΥ?(–¥≥ωΦΤΥψΙΐ≥ΧΘ§ΦΤΥψΫαΙϊΨΪ»ΖΒΫ0.1 g)
(Ω…Ρή”ΟΒΫΒΡœύΕ‘‘≠Ή”÷ ΝΩΘΚHΓΣ1 CΓΣ12 OΓΣ16 ClΓΣ35.5 CaΓΣ40)
ΓΨΧβΡΩΓΩ»γ±μ « NaClΓΔNH4Cl ‘Ύ≤ΜΆ§Έ¬Ε» ±ΒΡ»ήΫβΕ»ΓΘ
Έ¬Ε»/Γφ | 0 | 20 | 40 | 60 | 80 | |
»ήΫβΕ»/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
NH4Cl | 29.4 | 37.2 | 45.8 | 55.2 | 65.6 | |
Θ®1Θ©“‘…œΝΫ÷÷Έο÷ ΒΡ»ήΫβΕ» ήΈ¬Ε»”ΑœλΫœ¥σΒΡ «_____ΓΘ
Θ®2Θ©40Γφ ±Θ§ΫΪ 40.6g NaCl Φ”»κΒΫ 100g Υ°÷–Θ§≥δΖ÷ΫΝΑη Ι÷°»ήΫβΘ§ΥυΒΟ»ή“ΚΒΡ÷ ΝΩΈΣ_____gΓΘ
Θ®3Θ©20Γφ ±Θ§NaCl ±ΞΚΆ»ή“ΚΒΡ»ή÷ ÷ ΝΩΖ÷ ΐΈΣΘ®ΨΪ»ΖΒΫ–Γ ΐΒψΚσ 1 ΈΜΘ©_____ΓΘ
ΓΨΧβΡΩΓΩ»ΐ÷÷Φ“ΆΞ≥Θ”Ο«εΫύΦΝΒΡ–≈œΔ»γœ¬±μΥυ Ψ:
«εΫύΦΝ |
|
|
|
Οϊ≥Τ | Ϋύ≤όΝι | ≤ Τ·“Κ | Τ·ΑΉΥ° |
”––ß≥…Ζ÷ | HCl | H2O2 | NaClO Θ®¥Έ¬»ΥαΡΤΘ© |
Θ®1Θ©Ϋύ≤όΝιΩ…”Ο”Ύ€[≥ΐΥ°ΙΗΘ®÷ς“Σ≥…Ζ÷ΈΣCaCO3Θ©Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________ΓΘ
Θ®2Θ© Ι”Ο≤ Τ·“Κ ±Ιΐ―θΜ·«βΖ÷Ϋβ ΆΖ≈≥ωΜν–‘―θΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________ΓΘ
Θ®3Θ©Ϋύ≤όΝιΚΆΤ·ΑΉΥ°ΜλΚœΚσΘ§HCI”κNaCl0ΜαΖΔ…ζΖ¥”ΠΘ§≤ζ…ζ”–ΕΨΤχΧ嬻ΤχΘ®Cl2Θ©Θ§Ά§ ±…ζ≥…Υ°ΚΆ”…ΝΫ÷÷‘ΣΥΊΉι≥…ΒΡ―ΈΘ§ΗΟ―Έ «______________ΓΘ
ΓΨΧβΡΩΓΩΡ≥―–ΨΩ–ΓΉιΖΔœ÷Θ§ΓΑΈ§C≈ίΧΎΤ§Γ±(±ΘΫΓΤΖΘ§÷ς“Σ≥…Ζ÷ΦϊΆΦ1)»ή”ΎΥ°÷–Θ§”––μΕύΤχ≈ί≤ζ…ζ(»γΆΦ2)ΓΘΗΟ–ΓΉιΆ§―ßΫχ––ΝΥ»γœ¬ΧΫΨΩΓΘ

Θ®ΧΫΨΩΡΎ»ίΘ©ΗΟΤχΧεΒΡ≥…Ζ÷
Θ®≤¬œκ”κΦΌ…ηΘ©
–ΓΜΣΥΒΘΚΗΟΤχΧεΩ…ΡήCO2ΓΔO2ΓΔCOΓΔH2ΓΔN2ΓΘ
–ΓΟςΥΒΘΚ≤ΜΩ…ΡήΚ§”–N2Θ§“ρΈΣ__________ΓΘ
–ΓΖΦΥΒΘΚ≤ΜΩ…ΡήΚ§”–COΚΆH2Θ§“ρΈΣ¥”“©ΤΖΑ≤»ΪΫ«Ε»ΩΦ¬«Θ§H2“Ή»Φ“Ή±§Θ§CO_____ΓΘ
ΗΟ–ΓΉιΆ§―ß»œΈΣΘΚΗΟΤχΧεΩ…ΡήΚ§”–CO2ΓΔO2÷–ΒΡ“Μ÷÷ΜρΝΫ÷÷ΓΘ
Θ®Ϋχ–– Β―ιΘ©
Β―ι±ύΚ≈ | Β―ι≤ΌΉς | Β―ιœ÷œσ |
ΔΌ | ΫΪΤχΧεΆ®»κ≥Έ«ε ·Μ“Υ°÷– | ≥Έ«ε ·Μ“Υ°±δΜκΉ« |
ΔΎ | ΫΪ¥χΜπ–«ΒΡΡΨΧθ…λ»κΗΟΤχΧε÷– | ¥χΜπ–«ΒΡΡΨΧθΟΜ”–Η¥»Φ |
Θ®ΒΟ≥ωΫα¬έΘ©(1)”… Β―ιΔΌΩ…÷ΣΘ§ΗΟΤχΧε÷–ΩœΕ®Κ§”–_____Θ§–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_______ΓΘ
(2)”… Β―ιΔΎ_____ (ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±)»ΖΕ®ΗΟΤχΧε÷–≤ΜΚ§―θΤχΘ§άμ”… «______ΓΘ