��Ŀ����
ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ

��1������ǡ����ȫ��Ӧʱ������CO2������Ϊ g
��2���������Ʒ�к����ʵ����������Ƕ��٣�������������һλС������ͬ��
��3�����㵱�����봿��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�

��1������ǡ����ȫ��Ӧʱ������CO2������Ϊ g
��2���������Ʒ�к����ʵ����������Ƕ��٣�������������һλС������ͬ��
��3�����㵱�����봿��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
��1��4.4g��2��11.7% ��3��16.3%
�����������1��������Ʒ�е���Ҫ�ɷ�Ϊ̼���ƣ��������ᷴӦ���ɶ�����̼���塣����ͼʾ��֪��������73gϡ����ʱ����������������ﵽ�����ֵ������ʱ̼����ǡ����ȫ��Ӧ������ǡ����ȫ��Ӧʱ���ɶ�����̼������Ϊ4.4g��
��2�����������֪����֪��Ϊ������̼��������δ֪��Ϊ��Ʒ�к����ʵ���������������˼·Ϊ���ɸ��ݷ�Ӧ�ж�����̼��̼���Ƶ�������ϵ���̼���Ƶ���������һ���������Ʒ�к��Ȼ��Ƶ�����������
��3�����������֪����֪��Ϊ������̼��������δ֪��Ϊ������Һ�����ʵ���������������˼·Ϊ��������ҺΪ�Ȼ�����Һ���ɸ��ݷ�Ӧ�ж�����̼���Ȼ��Ƶ�������ϵ��������Ȼ��Ƶ��������ټ�����Ʒ��ԭ���Ȼ��Ƶ���������Ϊ������Һ�е����ʵ��������ٸ��������غ㶨�ɿ����������Һ�����������ɼ����������Һ�����ʵ���������������������£�
��2���⣺����Ʒ�к�̼���Ƶ�����Ϊx����Ӧ�����Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl==2NaCl+H2O+CO2��
106 117 44
x y 4.4g
106��44=x��4.4g
x=10.6g
117��44=y��4.4g
y=11.7g
��Ʒ�к����ʵ���������Ϊ��
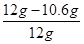 ��100%��11.7%
��100%��11.7%��3��������Һ�����ʵ���������Ϊ��
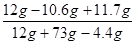 ��100%��16.3%
��100%��16.3%�𣺣�1�����ɶ�����̼������Ϊ4.4g��
��2����Ʒ�к����ʵ���������Ϊ11.7%��
��3��������Һ�����ʵ���������Ϊ16.3%��

��ϰ��ϵ�д�
 ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ

