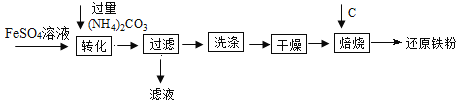
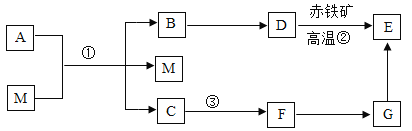
题目内容
【题目】氢氧化钠固体,因不慎敞口放置了一段时间,已经部分变质.化学课外兴趣小组的同学决定测定该瓶 试剂变质的程度,他们在知识回顾的基础上,依次进行了如下的实验操作:
(知识回顾)
氢氧化钠必须密封保存,理由是:①氢氧化钠固体会吸收水分而潮解;②氢氧化钠与二氧化碳反应变质, 发 生反应的化学方程式是: 。
(实验操作)
第一步:取该瓶中的试剂20g加水配制成溶液;
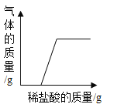
第二步:向上述溶液中加入足量的稀盐酸,反应生成气体的质量变化如图所示。

根据实验步骤和图像进行分析与计算:
(1)20g试剂与稀盐酸反应产生二氧化碳的质量 g.
(2)计算该20g试剂中含有杂质Na2CO3的质量分数(写出计算过程)。(结果精确到 0.1%)
(3)如果第二步改为向第一步配制成的溶液中加入足量的澄清石灰水,则生成沉淀的质量为 g。
【答案】![]() ; 2.2;26.5%;5
; 2.2;26.5%;5
【解析】
氢氧化钠必须密封保存,氢氧化钠与二氧化碳反应产生碳酸钠和水的化学方程式是:![]() 。
。
解:(1)由图像可知:20g试剂与稀盐酸反应产生二氧化碳的质量2.2g;
(2)设:20g试剂中含Na2CO3的质量为x
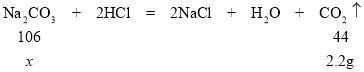
![]()
![]()
![]()
答:20g试剂中含有杂质 Na2CO3的质量分数是26.5%。
(3)设:生成沉淀的质量为y

![]() y=5.0g
y=5.0g
答:生成碳酸钙沉淀的质量为5.0g。

【题目】(1)小婷和小彤在帮助老师整理药品时,发现有四瓶失去标签的溶液,只知道它们分别是氢氧化钙溶液、硫酸铜溶液、碳酸钠溶液和稀盐酸。小婷很快就判断岀其中瓶是硫酸铜溶液。小彤把其他三种溶液分别编号成A、B、C,然后两两混合进行如下实验:
实验 | A+B | B+C | A+C |
现象 | 无明显现象 | 有白色沉淀产生 | 有气体产生 |
据此,小彤很快就分辨出了三种未知溶液。回答下列问题:
①小婷判断出硫酸铜溶液的依据是_________________。
②A与B反应的基本类型是___________。
③C的化学式是___________。
(2)如图是Fe及其化合物的化合价——物质类别二维图。
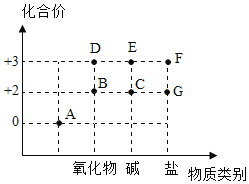
①A物质所属物质类别是___________(填“化合物”或“单质”)。
②C在潮湿的空气中很容易发生化合反应变成E,该反应的化学方程式是_________________________。