��Ŀ����
����Ŀ��(20��)ijFe3O4��Ʒ�п��ܺ��е�������Fe2O3��FeO���е�һ�֡�Ϊ̽�����ʵ����������·�����
��ʵ����ơ�ͬѧ�dz�ȡ��23.28 g��Fe3O4��Ʒ��ͼ�ٽ���������ʵ�飺

��ʵ���������̽��˼·��ͬ����ȤС��ֳɼס��ҡ�����������С�顣
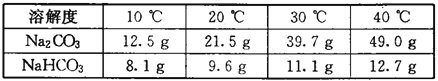
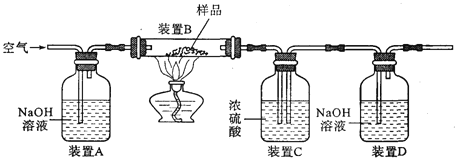
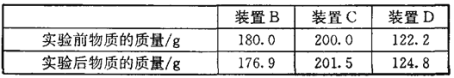
���飻��1������ͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯������ȷ�ϸ�Fe3O4��Ʒ�е����ʡ�B�е�����Լ���______������ţ���
�ٳ���ʯ��ˮ ����������Ũ��Һ ��ϡ���� ��ˮ
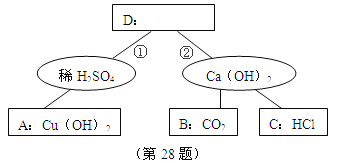
��2������ʵ������У�CO��������_______ ������ţ���
��CO������Ϊ��Ӧ��
��ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը
��ֹͣ���Ⱥ�ֹA�������ﱻ����
��ֹͣ���Ⱥ��ֹB�е���Һ������A��
�ݶ�B�з�Ӧ����һ���Ľ������ã�����CO2�뷴ӦҺ��ֽӴ�����Ӧ
���飺
��3������ͬѧ��ΪBװ�÷�Ӧǰ����������ƫС��Ӱ����㡢����������Ϊ���ǵ�������_______ ���Ľ�Ϊ��Bװ�ú��һʢ��_______�����Լ����ƣ��Ĺ��ƿ����ˮװ�ã���Bװ�ú�װ�á����������Ӷ���С��
���飺
��4������ͬѧ��Bװ����Һ��Ļ�ΪBa(OH)2��Һ��ͨ���������ɵ�_______���ѧʽ��������������������⡣Ϊ�˻�ó���������ͬѧ��Ҫ���еIJ����й��ˡ�_______��________��������
���������ϡ�
Fe2O3��CO��Ӧ�����¶����߶����еģ�������Fe3O4��������FeO(��ɫ)���������Fe�����������ⶨ�����Ƴɷ�Ӧ������Aװ���в������ڵĹ����������¶ȵı仯���ߣ���ͼ�ڣ���
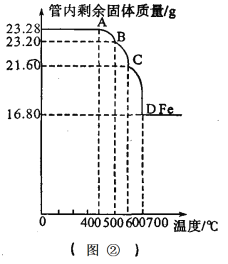
��5��д��BC�η�����Ӧ�Ļ�ѧ����ʽ��______________ ��
��6��ȷ����Fe3O4��Ʒ�к��е�������_______�����к�Fe3O4��������_______g������Ҫд�����̣�����֪Fe3O4��FeO��Fe2O3����Ԫ�ص����������ֱ�Ϊ72.4%��77.8%��70.0%)��
���𰸡���1���� ��2���٢ڢۢܢ�
��3��CO��B����Һ���ݳ�ʱ���������ˮ���� Ũ����
��4��BaCO3 ϴ�� ���� ��5��Fe3O4��CO����3FeO��CO2 ��6��Fe2O3 21
��������
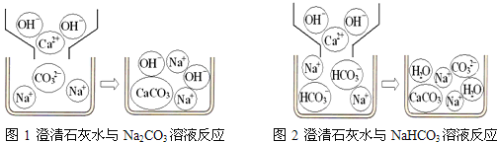
�����������1������ͬѧ��ͨ��Bװ�÷�Ӧǰ��������仯������ȷ�ϸ�Fe3O4��Ʒ�е����ʣ���ôBװ�õ���ҪĿ�ľ������շ�Ӧ���ɵĶ�����̼����B�е�����Լ����������ó���ʯ��ˮ��ԭ�����������Ƶ��ܽ�Ⱥ�С������Ч����
��2������ʵ������У�CO�������У���CO������Ϊ��Ӧ���ȷ����ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը����ȷ����ֹͣ���Ⱥ�ֹA�������ﱻ��������ȷ����ֹͣ���Ⱥ��ֹB�е���Һ������A�У���ȷ������B�з�Ӧ����һ���Ľ������ã�����CO2�뷴ӦҺ��ֽӴ�����Ӧ����ȷ����ѡ�٢ڢۢܢ�
��3������ͬѧ��ΪBװ�÷�Ӧǰ����������ƫС��Ӱ����㡢���������ǵ������ǣ�CO��B����Һ���ݳ�ʱ���������ˮ������ʹ��������ƫС������Ũ���������ˮ�ԣ��ʿ��Ľ�Ϊ��Bװ�ú��һʢ��Ũ�����Ĺ��ƿ����ˮװ�ã���Bװ�ú�װ�á����������Ӷ���С���
��4������ͬѧ��Bװ����Һ��Ļ�ΪBa(OH)2��Һ����ô������Ӧ��CO2 +Ba(OH)2 ==BaCO3��+ H20���ʿ�ͨ���������ɵ�BaCO3������������������⡣Ϊ�˻�ó���������ͬѧ��Ҫ���еIJ����й��ˡ�ϴ��������������
��5���������ϣ�Fe2O3��CO��Ӧ�����¶����߶����еģ�������Fe3O4��������FeO(��ɫ)���������Fe ����BC�η�����Ӧ�Ļ�ѧ����ʽ��Fe3O4��CO����3FeO��CO2
��6�����������غ㶨�ɣ���Ӧ������õ���������Ϊ16.8g��˵��ԭ��Ʒ�к���Ԫ�ص�����ҲΪ16.8g ������Ԫ�ص���������=16.8g/23.28g��100%=72.2%�����š��м�ֵ��ƽ��ֵ����˼�룬Fe3O4����Ԫ�ص���������=77.8%>72.2%����ô��һ�����ʵ���Ԫ������������ҪС��72.2%����Fe3O4��Ʒ�к��е�������Fe2O3��������Fe3O4��������x����ôFe2O3�������ǣ�23.28g-x���������е�ʽ:
x��168/232��100%+��23.28g-x����112/160��100%���ɼ����x=21g

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�