��Ŀ����
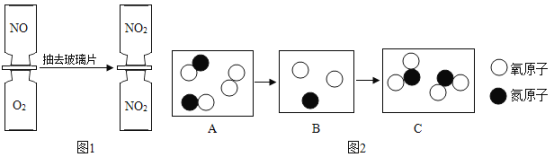
����Ŀ��С��Ϊ��̽��������һ��ʱ���ʯ��ʯ�и��ɷֵ��������������ʸ��²��ֽ⡢������ˮҲ�����ᷴӦ������������ͼ��ʾ��ʵ�飺
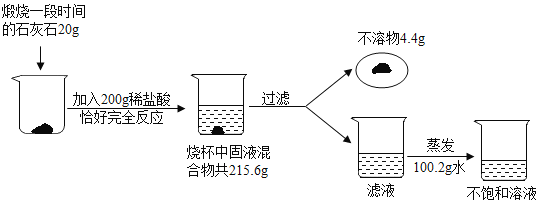
��ش��������⣺
��1��д�����������ɵĻ�ѧ��Ӧ����ʽ______________________��
��2�����������������___________��
��3��д�������̽���Ĺ�����̼���������X���ı���ʽ__________��
��4�������պ��ʯ��ʯ�и��ɷֵ�����������________��
��5���������õIJ�������Һ�У����ʵ���������Ϊ________��
��6��������40t������һ��ʱ���ʯ��ʯ������������գ����Եõ������ʵ���ʯ�ҵ�������__________��
���𰸡�CaCO3+2HCl=CaCl2+H2O+CO2�� 4.4g ![]() 25��14��11 20% 31.2t
25��14��11 20% 31.2t
��������
��1��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��2�����ɶ�����̼������Ϊ��20g+200g-215.6g=4.4g��
��3���������̼�������ΪX�������Ȼ��Ƶ�����Ϊy
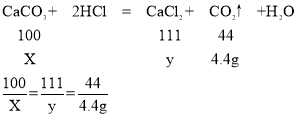
X=10g
y=11.1g
��4��̼����ڸ��µ����������������ƺͶ�����̼����ѧ����ʽΪ��
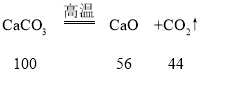
�������պ��ʯ��ʯ�и��ɷֵ�����������100��56��44=25��14��11��
��5�����Ի���������Ƶ�����Ϊ��20g-10g-4.4g=5.6g
�������������Ȼ��Ƶ�����Ϊz
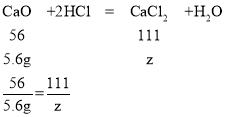
z=11.1g
�����������õIJ�������Һ�У����ʵ���������Ϊ��![]() ��100%=20%��
��100%=20%��
��6��40tʯ��ʯ�к���̼�������Ϊ��40t��![]() ��100%=20t
��100%=20t
�����ɶ�����̼������Ϊm
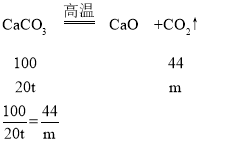
m=8.8t
���Կ��Եõ������ʵ���ʯ�ҵ�������40t-8.8t=31.2t��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����һ�������Ҵ�C2H6O����������һ����ȫ��յ���������ȼ����÷�Ӧǰ�����ʵ��������±��������ж���ȷ���ǣ�������
���� | C2H6O | O2 | CO2 | H2O | X |
��Ӧǰ����/g | 4.6 | 8 | 0 | 0 | 0 |
��Ӧ������/g | 0 | 0 | 4.4 | 5.4 | m |
A.���� m ��ֵΪ 3.8
B.X �����Ǹ÷�Ӧ�Ĵ���
C.X ���ܺ�����Ԫ��
D.����ʼʱ������������ 9.6g������ X ����