��Ŀ����
����Ŀ����һ������̼���ƺ�̼�����ƵĻ���Ϊ�˲ⶨ�京��������ԱСӱȡ19g��Ʒ�����ձ��У�����100gˮ����ܽ����ε���ϡ���Ტ���Ͻ��裬��B��ʱǡ����ȫ��Ӧ������ձ�����Һ�������������ϡ���������Ĺ�ϵ������ͼ��ʾ����Ӧ���ɵ�����ȫ���ݳ�������֪̼������ϡ���ᷴӦ���������У�
��һ����Ӧ��Na2CO3+HCl=NaCl+NaHCO3
�ڶ�����Ӧ��NaHCO3+HCl=NaCl+H2O+CO2��
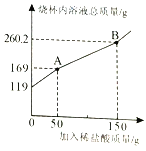
����1��ͼ��AB�α�ʾ�� ����Ӧ����Ӧ�����ɵĶ�����̼������ g��
��2���������ϡ�������������������Ƕ��٣�
��3����Ʒ��̼�����Ƶ������Ƕ��٣�
���𰸡���1���� 8��8 ��2��7��3% ��3��8��4g
��������
�����������1������̼������ϡ������������еķ�Ӧ��������һ����Ӧ��û�в������壬�ڶ�����ʼ�������壬��ͼ��AB�α�ʾ��������Ӧ�����������غ㶨�ɣ���Ӧ�����ɵĶ�����̼����=119g+150g-260��2g= 8��8g
��2�����ݻ�ѧ����ʽ��NaHCO3+HCl=NaCl+H2O+CO2����CO2��HCl��������ϵ���ɼ������NaHCO3��Ӧ��HCl��������һ������ϡ������������������
�⣺��HCl����Ϊx
NaHCO3+HCl=NaCl+H2O+CO2��
36��5 44
x 8��8g
36��5��44=x:8��8g
x=7��3g
ϡ������������������=7��3g/100g��100%=7��3%
��3������ͼ���֪����һ����Ӧ���ĵ���������Ϊ50g������HCl����=50g��7��3%=3��65g��Ȼ���ٸ��ݵ�һ����Ӧ��Na2CO3+HCl=NaCl+NaHCO3��HCl��Na2CO3��������ϵ�������Na2CO3������
�⣺��Na2CO3������Ϊy
Na2CO3+HCl=NaCl+NaHCO3
84 36��5
y 3��65g
84��36��5=y:3��65g
y=8��4g

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
