��Ŀ����
��������������벻���������ϣ�
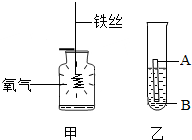
��1����������ͭ��ʹ�ñȽϹ㷺�Ľ�����
����ͼ1��ʾ��Ʒ�У����ý��������Ե��� ����չ�Ե��� ������ĸ��ţ���
�������кܺõĿ���ʴ���ܣ�ԭ���� ���û�ѧ����ʽ��ʾ����
��2����������Ǧ�Ͻ𣩳����ں��ӵ���Ԫ�����±���һЩ�������۵����ݣ�
����Ǧ�Ͻ���ij�ֽ���������������Ͻ���۵�����ͼ2��ʾ�Ĺ�ϵ��
���к������ʾ���� ����������������ĸ��ţ���
A���� B��Ǧ C����ȷ��

�ڱ���˿���顢Ǧ����������ɣ����۵�ԼΪ ������ĸ��ţ���
A��15��30��B��60��80��C��231��328��
��3�������ѣ�Ti���ĺϽ�㷺���ں��ա����켰��ѧ��ҵ����ҵ��ұ�������ѵĹ��������ѿ�ʯ����Ҫ�ɷ�Ϊ������������ѧʽΪFeTiO3������̿������Ϊԭ�ϣ��ڸ�����������ȡTiCl4���䷴Ӧ�Ļ�ѧ����ʽΪ��2FeTiO3+6C+7Cl2
2TiCl4+2X+6CO��Ȼ����þ��TiCl4���ڸ��������·�Ӧ���ɽ����Ѻ��Ȼ�þ��
�����������Ϣ�ش��������⣺
��FeTiO3����Ԫ�صĻ��ϼ�Ϊ �� �����Ʊ�TiCl4�ķ�Ӧ�У�X�Ļ�ѧʽΪ ��
��þ��TiCl4��Ӧ�Ļ�ѧ����ʽΪ ��
��4����ͭ����ƻƽ��ʵؼ�Ӳ����¯��ʯ��ZnCO3������ͭ��Cu2O����ľ̿�ۻ�Ϻ���ȵ�Լ800�棬�õ�һ��п��ͭ�ĺϽ�ͭ�������Ļ�ѧ��Ӧ�У�
ZnCO3
ZnO+CO2����2Cu2O+C
4Cu+CO2����2ZnO+C
2Zn+CO2��
��ԭ���к�72�ֳ�ͭ����ַ�Ӧ�����������û�ͭ��ͭ������Ϊ���٣���д��������̣�
��1����������ͭ��ʹ�ñȽϹ㷺�Ľ�����
����ͼ1��ʾ��Ʒ�У����ý��������Ե���
�������кܺõĿ���ʴ���ܣ�ԭ����
��2����������Ǧ�Ͻ𣩳����ں��ӵ���Ԫ�����±���һЩ�������۵����ݣ�
| ���� | �� | Ǧ | �� | �� |
| �۵�/�� | 231.9 | 327.5 | 271.3 | 320.9 |
���к������ʾ����
A���� B��Ǧ C����ȷ��

�ڱ���˿���顢Ǧ����������ɣ����۵�ԼΪ
A��15��30��B��60��80��C��231��328��
��3�������ѣ�Ti���ĺϽ�㷺���ں��ա����켰��ѧ��ҵ����ҵ��ұ�������ѵĹ��������ѿ�ʯ����Ҫ�ɷ�Ϊ������������ѧʽΪFeTiO3������̿������Ϊԭ�ϣ��ڸ�����������ȡTiCl4���䷴Ӧ�Ļ�ѧ����ʽΪ��2FeTiO3+6C+7Cl2
| ||
�����������Ϣ�ش��������⣺
��FeTiO3����Ԫ�صĻ��ϼ�Ϊ
��þ��TiCl4��Ӧ�Ļ�ѧ����ʽΪ
��4����ͭ����ƻƽ��ʵؼ�Ӳ����¯��ʯ��ZnCO3������ͭ��Cu2O����ľ̿�ۻ�Ϻ���ȵ�Լ800�棬�õ�һ��п��ͭ�ĺϽ�ͭ�������Ļ�ѧ��Ӧ�У�
ZnCO3
| ||
| ||
| ||
��ԭ���к�72�ֳ�ͭ����ַ�Ӧ�����������û�ͭ��ͭ������Ϊ���٣���д��������̣�
��������1�����ݽ��������ʽ��н��
��2���ٸ��ݺ�������������Ϊ��ʱ�۵�Ҫ����������Ϊ1ʱ�۵�߿��DZ��⣻�ڸ��ݺϽ���۵�������ɳɷֵ��۵�ȽϿ��ǣ�
��3���ٸ������������Ļ�ѧʽ��FeTiO3������������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�����ѵĻ��ϼۣ�
�ڸ��ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ������X�Ļ�ѧʽ��
�۸���������Ϣ��ϻ�ѧ����ʽ����д������
��4�����ݳ�ͭ���������û�ѧ��ʽʽ���������ͭ��ͭ��������
��2���ٸ��ݺ�������������Ϊ��ʱ�۵�Ҫ����������Ϊ1ʱ�۵�߿��DZ��⣻�ڸ��ݺϽ���۵�������ɳɷֵ��۵�ȽϿ��ǣ�
��3���ٸ������������Ļ�ѧʽ��FeTiO3������������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�����ѵĻ��ϼۣ�
�ڸ��ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ������X�Ļ�ѧʽ��
�۸���������Ϣ��ϻ�ѧ����ʽ����д������
��4�����ݳ�ͭ���������û�ѧ��ʽʽ���������ͭ��ͭ��������
����⣺��1������ͼ1��ʾ��Ʒ�У��������Ĵ����Կ����Ƴɹ������ý�������չ�ԣ������Ƴ����������ý����ĵ����Կ��Խ�ͭ�������ߣ����A��C��
�������кܺõĿ���ʴ���ܣ�ԭ�������ڿ����л��������Ӧ�γ����ܵ������ﱡĤ������������һ����������Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2�T2Al2O3�����4Al+3O2�T2Al2O3��
��2���ٺ�������������Ϊ��ʱ�۵�Ҫ����������Ϊ1ʱ�۵�ߣ���ΪǦ���۵�������۵�ߣ����Ժ������ʾ�����������������A��
�ںϽ���۵��������ɳɷ��۵�Ҫ�ͣ��顢Ǧ�������������������۵���͵���231.9������Ҫѡ���۵��231.9�ͣ��Ҳ��ܺܵͣ�����Ҫ�������£�����ѡ��B�����B��
��3�������������Ļ�ѧʽΪFeTiO3��������Ԫ�صĻ��ϼ�Ϊ+2�ۣ���Ԫ�صĻ��ϼ�Ϊ-2�ۣ�������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�����ѵĻ��ϼ�Ϊx����+2��+x+��-2����3=0�����x=+4��
�ʴ�Ϊ��+4��
�ڹ۲�÷�Ӧ2FeTiO3+6C+7Cl2
2TiCl4+2X+6CO����֪��Ӧǰ��ԭ����2������ԭ����2������ԭ����6����̼ԭ����6������ԭ����14������Ӧ���2X�⣬��ԭ����2������ԭ����8����̼ԭ����6������ԭ����6�������ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬2X��Ӧ��6����ԭ�Ӻ�2����ԭ�ӣ����X�Ļ�ѧʽΪFeCl3��
�ʴ�Ϊ��FeCl3��
�۸���������Ϣ��þ��TiCl4�ڸ��������·�Ӧ���ɽ����Ѻ��Ȼ�þ���÷�Ӧ�Ļ�ѧ����ʽΪTiCl4+2Mg
Ti+2MgCl2��
�ʴ�Ϊ��TiCl4+2Mg
Ti+2MgCl2��
��4�������������û�ͭ��ͭ������Ϊx��
2Cu2O+C
4Cu+CO2��
288 256
72t x
=
x=64t
�����������û�ͭ��ͭ������Ϊ64t��
�������кܺõĿ���ʴ���ܣ�ԭ�������ڿ����л��������Ӧ�γ����ܵ������ﱡĤ������������һ����������Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2�T2Al2O3�����4Al+3O2�T2Al2O3��
��2���ٺ�������������Ϊ��ʱ�۵�Ҫ����������Ϊ1ʱ�۵�ߣ���ΪǦ���۵�������۵�ߣ����Ժ������ʾ�����������������A��
�ںϽ���۵��������ɳɷ��۵�Ҫ�ͣ��顢Ǧ�������������������۵���͵���231.9������Ҫѡ���۵��231.9�ͣ��Ҳ��ܺܵͣ�����Ҫ�������£�����ѡ��B�����B��
��3�������������Ļ�ѧʽΪFeTiO3��������Ԫ�صĻ��ϼ�Ϊ+2�ۣ���Ԫ�صĻ��ϼ�Ϊ-2�ۣ�������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0�����ѵĻ��ϼ�Ϊx����+2��+x+��-2����3=0�����x=+4��
�ʴ�Ϊ��+4��
�ڹ۲�÷�Ӧ2FeTiO3+6C+7Cl2
| ||
�ʴ�Ϊ��FeCl3��
�۸���������Ϣ��þ��TiCl4�ڸ��������·�Ӧ���ɽ����Ѻ��Ȼ�þ���÷�Ӧ�Ļ�ѧ����ʽΪTiCl4+2Mg
| ||
�ʴ�Ϊ��TiCl4+2Mg
| ||
��4�������������û�ͭ��ͭ������Ϊx��
2Cu2O+C
| ||
288 256
72t x
| 288 |
| 72t |
| 256 |
| x |
x=64t
�����������û�ͭ��ͭ������Ϊ64t��
�����������ؼ���Ҫ֪���Ͻ���۵��������ɳɷ��۵�ͣ�Ӳ�ȴ���������������غ㶨�ɻش����⣮

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ

 ��������������벻����������1���ڵؿ��ﺬ���ӵڶ�λ�Ľ���Ԫ����
��������������벻����������1���ڵؿ��ﺬ���ӵڶ�λ�Ľ���Ԫ����
 ��������������벻���������������ֽ��������֡�ʹ�õ��Ⱥ�˳������Ϊ��ͭ����������
��������������벻���������������ֽ��������֡�ʹ�õ��Ⱥ�˳������Ϊ��ͭ����������