��Ŀ����
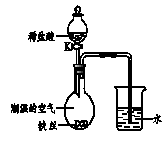
ij�о���ѧϰ�С�飬������װ����ѡ�ò���װ����װ����ʵ��������ȡ��������ͨ����ˮ�����ⶨ���ռ��������������
A B C D E F
��1��д��ͼ���б�ŵ����������ƣ��� ���� ���� ��
��2����ͬѧ��һ�������ĸ��������ȡ��������Ӧ�Ļ�ѧ����ʽΪ ������ʵ��װ�ýӿڵ���ȷ����˳��Ϊa�� �� �� ��
��3��ʵ������У���ͬѧ�۲쵽Eװ���е�ˮ��Ϊ�Ϻ�ɫ��������Ϊ ����Eװ�ò�����ˮ�е�Ե�ʡ�Ϊ�˷�ֹ��������г�������������Ӧ�ĸĽ���ʩ�� ��
��4��ʵ���������ͬѧ���ⷢ���ռ��������������������ֵ��Ϊ�ˣ����������������ֲ��룬���ڽ������̽����
����� ��
����� ��
����� ��
�������ϲ���������� ��
��1��������̨ �ڼ���ƿ ����Ͳ
��2��2KMnO4 K2MnO4��MnO2��O2�� f �� e �� g
K2MnO4��MnO2��O2�� f �� e �� g
��3��������� ���Թܿڷ�����
��4����������Ƿ�Ӧ���ɵĶ������̷ֽ�ų�����
��������Ƿ�Ӧ���ɵ�����طֽ�ų�����
�������������غͶ������̹�ͬ�ų�����
�������ϲ���������ǣ�����غͶ������̶�������Ԫ��
���������������1������������ʶ��
��2���ø��������ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4 K2MnO4��MnO2��O2������ΪҪͨ����ˮ�����ⶨ���ռ������������������Ӧ��Eװ�����ռ������������ų���ˮ������Ͳ�ڣ��������ӵ�˳��Ϊ��f �� e �� g
K2MnO4��MnO2��O2������ΪҪͨ����ˮ�����ⶨ���ռ������������������Ӧ��Eװ�����ռ������������ų���ˮ������Ͳ�ڣ��������ӵ�˳��Ϊ��f �� e �� g
��3�������������ˮ��ˮ��Һ���Ϻ�ɫ������ʵ������У���ͬѧ�۲쵽Eװ���е�ˮ��Ϊ�Ϻ�ɫ��������Ϊ������ؽ���Eװ�ò�����ˮ�е�Ե�ʡ�Ϊ�˷�ֹ��������г�������������Ӧ�ĸĽ���ʩ�ǣ����Թܿڷ�����
��4�����������غ㶨�ɣ���ѧ��Ӧǰ���Ԫ����������������䣬Ҫ��Ӧ������������ô��Ӧ���б���Ҫ����Ԫ�أ����Կ��������¼�����룺
��������Ƿ�Ӧ���ɵĶ������̷ֽ�ų�����
��������Ƿ�Ӧ���ɵ�����طֽ�ų�����
�������������غͶ������̹�ͬ�ų�����
�������ϲ���������ǣ�����غͶ������̶�������Ԫ��
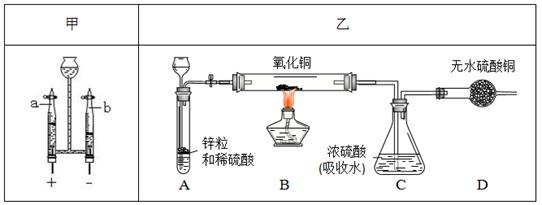
���㣺ʵ������ȡ�����װ�ã�ʵ���¹ʵķ�������

ˮ���˼�һ����������������ģ�Ϊ���������ᾭ�õĿɳ�����չ������Ӧ���˽�һЩ�й�ˮ��֪ʶ������ش�
��1����Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о���ˮ�̶���ߵķ����� ����ȥˮ�������Թ������ʵIJ����� ���˲�����Ҫ�õ����������������� ��
��2�������п��Բ��� ����Ӳˮ����ˮ������ͨ������ ������ˮ������ζ�����ʡ�
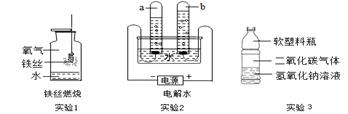
��3����ͼ�ǵ��ˮʵ��װ�á����й��ڵ��ˮʵ��������У���ȷ���� ������ţ���
| A����ʵ���֤��ˮ����Ԫ�غ���Ԫ����� |
| B���Թ�a���ռ������������� |
| C���Թ�a���Թ�b���ռ��������������������2:1 |
| D����ˮ�м�����ϡ�������ǿˮ�ĵ����� |
ˮ������ͨ�����������֮һ��
��1�������еġ�ˮ���кܶ��֡����С�ˮ�����ڴ�������� (����ĸ���)��
| A����ˮ | B������ˮ | C������ˮ | D����Ȫˮ |
��3����ͼΪ���ˮ��װ�ã�ͨ��һ��ʱ����Թ�2�����ռ�������Ϊ ��������Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ���� _������ϡ��ֽ⡱����Ӧ��


��4��ˮ��һ����Ҫ�Ļ���ԭ�ϡ��ȼҵͨ����ⱥ���Ȼ�����Һ�ķ�����ȡ�ռNaOH����ͬʱ������������������Cl2�����䷴Ӧ�Ļ�ѧ����ʽΪ ��