��Ŀ����
����Ŀ���㸡�飨��Ҫ�ɷ��ǹ�̼���ƣ�2Na2CO3��3H2O2������ˮ����ֳ�г�����������������ˮ������Na2CO3��H2O2����Ѹ������ˮ����������ij����С������ͼװ��̽���ٽ�H2O2�ֽ�����ء�

���������ϣ�H2O2�����·ֽ������
NaCl����ˮ�����Na+��Cl-��
Na2CO3����ˮ�����Na+��CO32-��CO32-��ˮ����ʹ��Һ�ʼ��ԡ�
����������裩��.Na+�ٽ�H2O2�ֽ⡣��.��Һ�ļ��Դٽ�H2O2�ֽ⡣
������ʵ�飩
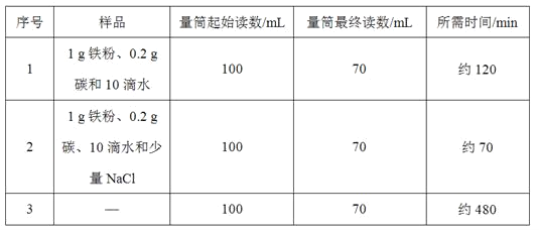
̽��һ���ֱ��������3��ʵ�飨80mL����ˮ�м���6.7g���㸡�顱��������Һ��H2O2��Ũ��ԼΪ4%����
ʵ�� | ʵ������ | ʵ���� | |
pH | �ܽ�����mg/L�� | ||
1 | �� 80 mL����ˮ | 6.96 | 5.68 |
�� �����㸡����6.7g | 9.89 | 7.97 | |
2 | �� 80 mL 4%��H2O2��Һ | 6.01 | 6.44 |
�� ������NaCl | 6.00 | 6.46 | |
�� �ټ�����Na2CO3 | 8.14 | 9.11 | |
3 | 80 mL 4%��H2O2��Һ�м�����NaOH��Һ | 9.26 | 10.97 |
����������ۣ�
��1������ʵ��1��ʵ���������롰�㸡�顱��Һ��ı仯��______��
��2��ͨ��ʵ��______��֤������1��������
��
̽��������5���ձ��зֱ����80mL4%��H2O2��Һ���ٷֱ�μ�NaOH��Һ����ò�ͬpH�µ��ܽ���������±���ʾ��
�ձ� | 1 | 2 | 3 | 4 | 5 |
pH | 8.00 | 8.59 | 9.29 | 10.43 | 11.47 |
�ܽ�����mg/L�� | 6.88 | 9.30 | 13.82 | 17.57 | 13.15 |
��4��̽�����ó��Ľ�����______��
����˼�����ۣ�
��5�����������ܼӿ�H2O2�ֽ����_______��
A MnO2 B NaCl C NaOH D Na2CO3
��6��̽��һ�У�ʵ��2-����ʵ��1-�ٶԱȣ��ܽ��������ԭ����______��
���𰸡�pH���ܽ������� 2 ʵ��2����̼���ơ�ʵ��3��������������Һ��pH�����ܽ������� ��pH��8.00~11.47֮��ʱ���ܽ������������� ACD �������ⳣ���·ֽ�ų�����
��������
��1������ʵ��1��ʵ���������롰�㸡�顱��Һ���pH���ܽ�������
��2��ʵ��2�м�������NaCl��ˮ�е��ܽ���û�����Ա仯��֤������1��������
��3��ʵ��2����̼���ơ�ʵ��3��������������Һ��pH�����ܽ������ӣ���֤������2������
��4������̽���������е����ݿ��Եó����ۣ���pH��8.00~11.47֮��ʱ���ܽ������������١�
��5��A��MnO2���Լӿ�H2O2�ֽ�����ʣ�����ȷ��
B������̽��һ�����ݿ�֪��NaCl��H2O2�ֽ�����ʼ���û��Ӱ�죬�ʴ���
C������̽��һ�����ݿ�֪��NaOH���Լӿ�H2O2�ֽ�����ʣ�����ȷ��
D������̽��һ�����ݿ�֪��Na2CO3���Լӿ�H2O2�ֽ�����ʣ�����ȷ����ѡACD��
��6��̽��һ�У�ʵ��2-����80 mL 4%��H2O2��Һ���ܽ���6.44mg/L��ʵ��1-����80 mL����ˮ���ܽ���5.68mg/L�����߶Աȣ��ܽ��������ԭ���ǣ��������ⳣ���·ֽ�ų�������

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д�����Ŀ��Ϊ̽������ͭ�������ֽ����Ļ��˳�������A��B����������
����A | ����B |
|
|
(1)����֤���ֽ������˳��ķ�����________(����A������B��)��
(2)����B�У�֤�����Ļ�Ա�ͭǿ��������___________��