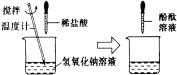��Ŀ����
����Ŀ����һ�û�ѧʵ����ϣ���ʦΪÿ��ͬѧ�ֱ��ṩ��һƿ����������Һ����������1����ϡ�������ⶨ�����ʵ����������������Ǽ���ͬѧ�����뼰������
��1������ͬѧ��ʵ����ͼ��ʾ�����ձ��м���5g����������Һ�����뼸�η�̪��Һ���õι���������1����ϡ���ᣬ�����Ͻ��裬����Һ��ɫǡ�ñ�Ϊ��ɫΪֹ��
��ش�
����̪��Һ��������________��
���ߵμ�ϡ���ᣬ��Ҫ�ò��������Ͻ����Ŀ����________��
������Һ��ɫǡ�ñ�Ϊ��ɫʱ������ȥ��ϡ����7.3g���������ƿ����������Һ�����ʵ���������Ϊ________��д��������̣�����ðٷ�����ʾ����
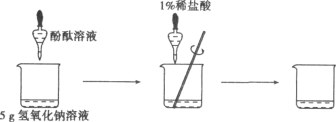
��2������ͬѧ�������ǣ����ձ��м���һ����������������Һ���õι���������1����ϡ���ᣬ�����Ͻ��裬ͨ����pH��ֽ��βⶨ��ҺpH�İ취���ﵽʵ��Ŀ�ģ�
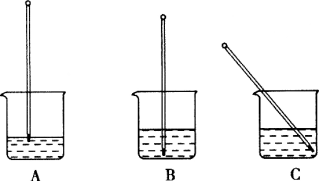
������ʹ��pH��ֽ�ķ�����ȷ����________������ĸ����
A����pH��ֽ���ɼ��ν�Լʹ��
B����pH��ֱֽ�Ӳ������Һ��
C����pH��ֽ���ڸɾ��İ״ɰ��ϣ��ò�����պȡ����Һ����pH��ֽ��
D����pH��ֽ��ʪ����ڲ���Ƭ�ϣ��ò�����պȡ����Һ����pH��ֽ��
��������pH��ֽ���βⶨ���Ϸ�������������ֵ������ȷ������ʦָ���£�����ͬѧȡ��5g����������Һ���������ֻ�ʵ�飬�ɼ��������������ʵ���������ҺpH�ı仯ͼ��ʾΪ��ͼ��ʾ����
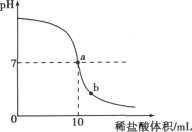
��ش�ͼ��a���ʾ�ĺ�����________��b����Һ�к��е���������________�������ӷ��ţ�������ͬѧҪ�����������ƿ����������Һ�����ʵ���������������Ҫ�õ����������⣬����Ϊ����Ҫ��������________��
��3������ͬѧ�ڵμ�ϡ����һ��ʱ�������Һ�����������ݲ�������һ���쳣���������������ǵ�̽��������ͨ��ʵ��֤���������õ���ƿ����������Һ�Ѿ����ֱ��ʣ�
���û�ѧ����ʽ��ʾ����ʵ�ԭ��________����Ҫ��ȥ��Һ�б������ɵ����ʣ����Ҫд�����ʵ�鷽����________��
���𰸡���1�����жϷ�Ӧ�Ƿ�ǡ����ɣ����ж��Ƿ���ȫ��Ӧ����
��ʹ��Ӧ���ֽӴ�����ȫ��Ӧ����ʹ��Ӧ���ֽӴ����ӿ췴Ӧ�ȣ�
���⣺7.3g��1����0.073g
���������Ƶ�����Ϊx
NaOH��HCl��NaCl��H2O
40 36.5
x 0.073g
40�U36.5��x�U0.073g
x��0.08g
0.08g��5g��100����1.6��
���ԣ�
��2����AC ��ǡ���к� Na����H�� ϡ������ܶ�
��3��CO2��2NaOH��Na2CO3��H2O ����������ʯ��ˮ��������������Һ��������
�����������𰸾��ɣ�
����������1����̪��������������Һ����Һ�ʺ�ɫ������ϡ���ᣬ��ɫ��dz���μӵ���ɫ����Ϊ��ɫ����ʾ����ǡ����ȫ��Ӧ����ͨ����̪����ɫ�仯ָʾ�����ǡ����ȫ��Ӧ��ʵ������ߵα߽��裬ʹ������ֽӴ�����Ӧ��ȫ����֪5gδ֪��������������NaOH��Һ��7.3g1����ϡ����ǡ����ȫ��Ӧ����ֱ����NaOH��Һ��������������Ϊx����
NaOH��HCl��NaCl��H2O
40 36.5
5g��x 7.3g��1��
![]() x��1.6��
x��1.6��
pH��ֽ����ֱ�Ӳ������Һ�У�Ҳ����������ʪ��ǰ����Ⱦ��Һ������ϡ����Һ������pH����pH�ı仯����ͼ��pH��7ʱ��ʾ�����ǡ����ȫ��Ӧ��b�����ѹ���������ΪNaCl��HCl��������ΪNa����H����ͼ����֪ϡ������������Ҫ֪��ϡ������ܶȲ��ܵó�ϡ�����������
��3��NaOH��Һ�ڿ�����������CO2���ʳ�Na2CO3����Na2CO3��ϡ���ᷴӦ�����ݲ�������ȥNaOH�е�Na2CO3�ɼ�������Ca��OH��2��Ba��OH��2��Һ���������������Ӻ���˳�ȥ��