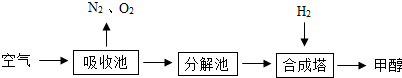
��Ŀ����
�ֲ�ö�����̼�ڿ����еĺ���Ϊ0.038%�����京������0.05%ʱ�ͻ�Ӿ�����ЧӦ��Ϊ��������⣬���������ŷ����⣬�п�ѧ�������������CO2�Ĺ��룺�ѿ�������̼�����Һ���ٴ���Һ����ȡ��CO2�����ںϳ�����ʹ֮��Ϊ�״���CH3OH����ˮ�����Ƴ��������ʹ�õij��ٽ�CO2��һ�ֽ�����̬��Һ̬֮���״̬�����ü����������£�
��˵�������ճ���ʢ�б��͵�K2CO3��Һ���ϳ����ڵķ�Ӧ����Ϊ300�桢200KPa�ʹ�������
��1���Գ��ٽ�CO2�����ǰ���õķ�����������������������Ի��������Ļ�����������
��2���ϳ����з�Ӧ�Ļ�ѧ����ʽΪ
��3��������������ͼ���ҳ�ѭ�����õ�������
��4������������������ճغͷֽ�أ�����ֱ��ͨ��ϳ���������Ϊ�����𣿲���������

��˵�������ճ���ʢ�б��͵�K2CO3��Һ���ϳ����ڵķ�Ӧ����Ϊ300�桢200KPa�ʹ�������
��1���Գ��ٽ�CO2�����ǰ���õķ�����������������������Ի��������Ļ�����������
����������
����������
����2���ϳ����з�Ӧ�Ļ�ѧ����ʽΪ
CO2+3H2
CH3OH+H2O
| ||
CO2+3H2
CH3OH+H2O
��
| ||
��3��������������ͼ���ҳ�ѭ�����õ�������
K2CO3ˮ
K2CO3ˮ
����4������������������ճغͷֽ�أ�����ֱ��ͨ��ϳ���������Ϊ�����𣿲���������
���У����ֱ��ͨ��ϳ��������ڿ�����CO2�ĺ���̫���ˣ����������ɼ״��ķ�Ӧ���У�ͬʱ�����������Ϻ��ڸ�����Ҳ�ᷢ����ը��
���У����ֱ��ͨ��ϳ��������ڿ�����CO2�ĺ���̫���ˣ����������ɼ״��ķ�Ӧ���У�ͬʱ�����������Ϻ��ڸ�����Ҳ�ᷢ����ը��
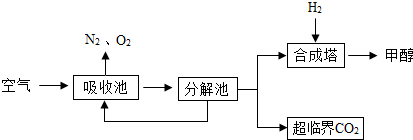
����������1��������̼����������ǶԳ�����ı�����
��2���������и������������Եó���ѧ����ʽ��
��3��������ͼ�����ҳ�ѭ�����õ����ʣ�
��4�������еĶ�����̼�ĺ����Ǻܵ͵ģ����Կ����еĶ�����̼����ֱ�����õģ������������������ϵ�ȼ�����ʱ���ܷ�����ը��
��2���������и������������Եó���ѧ����ʽ��
��3��������ͼ�����ҳ�ѭ�����õ����ʣ�
��4�������еĶ�����̼�ĺ����Ǻܵ͵ģ����Կ����еĶ�����̼����ֱ�����õģ������������������ϵ�ȼ�����ʱ���ܷ�����ը��
����⣺��1�����������ƻ������㣬�ö�����̼����������ܱ��������㣮
��2���������и�����������������̼����ֱ��������գ���������ּ����������ɼ״���ˮ�����Ի�ѧ����ʽΪ����CO2+3H2
CH3OH+H2O
��3�����ճ��еķ�ӦΪ��K2CO3+H2O+CO2=2KHCO3���ֽ���еķ�ӦΪ��2KHCO3
K2CO3+H2O+CO2�����ɼ�̼��غ�ˮ���ظ����ã�
��4�����ճ�ֻ�����˶�����̼���ֽ����ֻ�����˶�����̼һ�����壬����ͨ�����ճغͷֽ���з�����������Ӧ�����Գ�ȥ�������������壬�õ���������̼�����������п�ȼ�ԣ�������������Ȼᷢ����ը��
�ʴ𣻲��С����ֱ��ͨ��ϳ��������ڿ�����CO2�ĺ���̫���ˣ����������ɼ״��ķ�Ӧ���У�ͬʱ�����������Ϻ��ڸ�����Ҳ�ᷢ����ը��
�ʴ�Ϊ��
��1�����������㣻
��2��CO2+3H2
CH3OH+H2O��
��3��K2CO3��ˮ��
��4�����У����ֱ��ͨ��ϳ��������ڿ�����CO2�ĺ���̫���ˣ����������ɼ״��ķ�Ӧ���У�ͬʱ�����������Ϻ��ڸ�����Ҳ�ᷢ����ը��
��2���������и�����������������̼����ֱ��������գ���������ּ����������ɼ״���ˮ�����Ի�ѧ����ʽΪ����CO2+3H2
| ||
��3�����ճ��еķ�ӦΪ��K2CO3+H2O+CO2=2KHCO3���ֽ���еķ�ӦΪ��2KHCO3
| ||
��4�����ճ�ֻ�����˶�����̼���ֽ����ֻ�����˶�����̼һ�����壬����ͨ�����ճغͷֽ���з�����������Ӧ�����Գ�ȥ�������������壬�õ���������̼�����������п�ȼ�ԣ�������������Ȼᷢ����ը��
�ʴ𣻲��С����ֱ��ͨ��ϳ��������ڿ�����CO2�ĺ���̫���ˣ����������ɼ״��ķ�Ӧ���У�ͬʱ�����������Ϻ��ڸ�����Ҳ�ᷢ����ը��
�ʴ�Ϊ��
��1�����������㣻
��2��CO2+3H2
| ||
��3��K2CO3��ˮ��
��4�����У����ֱ��ͨ��ϳ��������ڿ�����CO2�ĺ���̫���ˣ����������ɼ״��ķ�Ӧ���У�ͬʱ�����������Ϻ��ڸ�����Ҳ�ᷢ����ը��
����������ͨ���о�������̼�Ի�����Ӱ�쿼��Զ�����̼���˽⣮�������е������Ի�ѧ����ʽ�������ǿ�����ص㣮

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
�����Ŀ