��Ŀ����
�ֲ�ö�����̼�ڿ����еĺ���Ϊ0.038%�����京������0.05%ʱ�ͻ�Ӿ�����ЧӦ����������һ�������������ŷ�������һ��������������������ã�
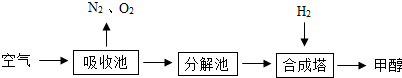
�п�ѧ�������������CO2�Ĺ��룺�ѿ�������̼�����Һ������Һ����ȡ��CO2�����ںϳ���ʹ֮��Ϊȼ�ϼ״���CH3OH����ˮ���ü����������£�

��˵�������ճ���ʢ�б��͵�K2CO3��Һ���ϳ����ڵķ�Ӧ����Ϊ300�桢200kPa�ʹ�������ʾ�������ճ��з����ķ�Ӧ��ѧ����ʽΪK2CO3+H2O+CO2=2KHCO3���ڷֽ����KHCO3�ֽ��ͷų�CO2��������K2CO3��H2O��
���ںϳ����з�Ӧ�Ļ�ѧ����ʽΪ
��������������ͼ���ҳ�ѭ�����õ�����
�п�ѧ�������������CO2�Ĺ��룺�ѿ�������̼�����Һ������Һ����ȡ��CO2�����ںϳ���ʹ֮��Ϊȼ�ϼ״���CH3OH����ˮ���ü����������£�

��˵�������ճ���ʢ�б��͵�K2CO3��Һ���ϳ����ڵķ�Ӧ����Ϊ300�桢200kPa�ʹ�������ʾ�������ճ��з����ķ�Ӧ��ѧ����ʽΪK2CO3+H2O+CO2=2KHCO3���ڷֽ����KHCO3�ֽ��ͷų�CO2��������K2CO3��H2O��
���ںϳ����з�Ӧ�Ļ�ѧ����ʽΪ
3H2+CO2
CH3OH+H2O
| ||
| 200kPa |
3H2+CO2
CH3OH+H2O
��
| ||
| 200kPa |
��������������ͼ���ҳ�ѭ�����õ�����
K2CO3
K2CO3
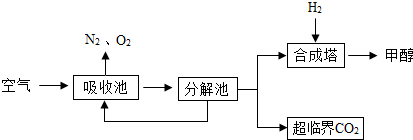
������������Ŀ��������Ϣ��֪��������̼���������Ƽ״������ճ���ʢ�б��͵�K2CO3��Һ�������ճ��з����ķ�Ӧ��ѧ����ʽΪK2CO3+H2O+CO2=2KHCO3���ڷֽ����KHCO3�ֽ��ͷų�CO2��������K2CO3��H2O��Ȼ���ںϳ����ڣ������Ͷ�����̼��300�桢200kPa�ʹ����������£����ɼ״���ˮ���ڴ˹����У�̼��ؿ���ѭ�����ã�
����⣺�������Ͷ�����̼��300�桢200kPa�ʹ����������£����ɼ״���ˮ����ƽ���ɣ��ʴ�Ϊ��3H2+CO2
CH3OH+H2O
�ڰѿ�������̼�����Һ������Һ����ȡ��CO2��K2CO3+H2O+CO2=2KHCO3���ڷֽ����KHCO3�ֽ��ͷų�CO2��������K2CO3��H2O�����̼��ؿ���ѭ�����ã��ʴ�Ϊ��K2CO3
| ||
| 200kPa |
�ڰѿ�������̼�����Һ������Һ����ȡ��CO2��K2CO3+H2O+CO2=2KHCO3���ڷֽ����KHCO3�ֽ��ͷų�CO2��������K2CO3��H2O�����̼��ؿ���ѭ�����ã��ʴ�Ϊ��K2CO3
�����������㿼����̼Ԫ�ص�ѭ����������̼�Ի�����Ӱ��ͻ�ѧ����ʽ����д�ȣ����̻�ͬѧ���ñ�֤�Ĺ۵㿴���⣮������Ŀ���з�ɢ��ѧϰ���Ƽ״��ķ�����һ��Ҫ��ǿ�����й�֪ʶ�㣬������Ļ����ϼ���Ӧ�ã���������Ҫ������������У�

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ