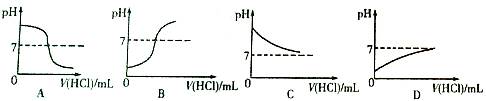
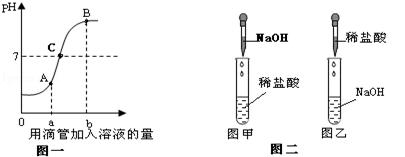
��Ŀ����
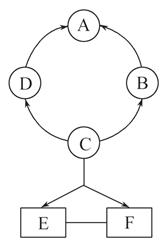
����п����ΪʳƷпǿ������ԭ�ϡ���ҵ�ϳ�����п����������п����п�����Ҫ�ɷ���ZnCO3������������Fe2O3 ��FeCO3 MgO�ȣ��������̼�ʾ��ͼ���£�
������ͼ�ش��������⣺
��1������п����ĥ�ɷ۵�Ŀ����_____��
��2��������Goethite�����Ե¹�ʫ�˸�£�Goethe�����������ģ����Ԫ����Fe��O��H����Է���������89������������ԭ�����U��ԭ�����U��ԭ����=______��
��3����д��������п��ϡ���ᷴӦ�Ļ�ѧ����ʽ____________________________���÷�Ӧ�Ļ���������______
��4������Һ3��֮ǰ����п�۵�Ŀ����______________
��5������Һ3��֮��IJ�������Ϊ______�����
��6������ͼ�����ݣ���п�����ZnCO3����������������________��100%��
��1����п����������ַ�Ӧ��
��2��1:2:1��
��3��Zn(OH)2+H2SO4= ZnSO4+2H2O�����ֽ⣻
��4����ȥʣ������
��5�������������ᾧ��
��6��25m2/13m1��125m2/65m1
���������������1������п����ĥ�ɷۣ����Ժ������ֽӴ����Ӷ�ʹ��п����������ַ�Ӧ��
��2������������Ԫ����Fe��O��H����Է���������89����ÿ������������ԭ�Ӹ���Ϊ1������ԭ�ӵĸ������ڵ�2��������Է�������Ҫ����89������ÿ����������ԭ�ӵĸ���Ϊx����ԭ�ӵĸ���Ϊy����16x+y+56=89���ɽ��x:y=2��1����������������ԭ�����U��ԭ�����U��ԭ����=1:2:1��
��3��������п��ϡ����ɷ������ֽⷴӦ��������п��ˮ����Ӧ�Ļ�ѧ����ʽΪZn(OH)2+H2SO4= ZnSO4+2H2O��
��4���ڳ���2�м����˹�����ϡ���ᣬ���ԡ���Һ3��֮ǰ����п�۵�Ŀ���������ᷴӦ��������п�Գ�ȥʣ������ᡣ
��5������Һ3��Ϊ����п��Һ����ͨ������������õ�����п���塣
��6������ͼʾ��֪����п�����ZnCO3�е�пԪ�ػ�ת��ΪZn(OH)2������Zn(OH)2��пԪ�ص������ɼ����̼��п�������������Ϊ 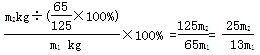
���㣺���������⡢���ݻ�ѧʽ�ļ���

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д���5�֣������±����ݣ��ش��������⡣
| ���� | �������� | �״� | ʳ��ˮ | ϴ�Ӽ� | ¯������ |
| pH | 1 | 3 | 7 | 9 | 12 |
��2��ˮ���е�ˮ������Ҫ�ɷ�Ϊ̼��ƺ�������þ���ð״� ����ܡ����ܡ���ȥ����
��3����������ϴ�Ӽ���ϴ�;��ϵ����ۣ������������� ��
��4������������¯������ ����ܡ����ܡ������ʹ�ã��ɱ���֪ʳ��ˮ�����ԣ��پٳ�һ�ֳ����Ե���Һ���� ��Һ��