��Ŀ����
����Ŀ�������ϵĽ�����Դ�㷺�ش����ڵؿǺͺ����С�
��1�����ý��������ԵIJ�ͬ�����Բ��ò�ͬ��ұ��������
ұ������ | ��Ҫԭ�� | �������� |
��ⷨ | ���ڵĽ�����������ͨ��������·ֽ� | �ơ��� |
�Ȼ�ԭ�� | ������������һ����̼����̼�ȣ��ڸ��µ������·�Ӧ | ����ͭ |
�ȷֽⷨ | �����������ڼ��ȵ������·ֽ� | ������ |
��1�����ý��������ԵIJ�ͬ�����Բ��ò�ͬ��ұ��������
�ٵ�����ڵ��Ȼ�þ���Եõ�þ���ֱ�д���Ȼ�þ��þ�Ļ�ѧʽ�����������þԪ�صĻ��ϼۣ�___________��___________��
��������ԭ��������һ����̼���������ķ�Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ��_________________________��
�۶��ٶ���ǰ���������о������ijɷ�ʱ�����Ⱥ�ɫ����������ĩ�õ�������ɫ�Ĺ���д���÷�Ӧ�Ļ�ѧ����ʽ��_______________________��
�ܴӺ���ǦԪ�ز��п��ɼ�ֵ�Ŀ�ʯ����ȡǦ��Ӧѡ�����������е�_______________��
��2��ij�������÷���м������ᷴӦ��ȡ�������������з�����9.8 t���������������Ϊ20%�����������ķ���м��Ӧ�����������������������Ƕ��٣���д��������̣�____________
���𰸡� ![]()
![]() 3CO+ Fe2 O3���� 2Fe + 3CO2 2HgO��2Hg + O2�� �Ȼ�ԭ�� 3.04t
3CO+ Fe2 O3���� 2Fe + 3CO2 2HgO��2Hg + O2�� �Ȼ�ԭ�� 3.04t
����������1���ٸ��ݻ�ѧʽ��Ԫ�ط��š����ϼ۵���д������д��
�ڢ۸��ݷ�Ӧ��������Ӧԭ�����
�ܸ��ݽ����Ļ�Ե�ǿ��������
��2��������ػ�ѧ����ʽ������
�⣺��1�����Ȼ�þ��þ�Ļ�ѧʽ�����������þԪ�صĻ��ϼۣ� ![]()
![]()
��3CO+ Fe2 O3���� 2Fe + 3CO2
��2HgO �� 2Hg + O2��
��Ǧ�Ļ��������֮ͭ�䣬�ʴӺ���ǦԪ�ز��п��ɼ�ֵ�Ŀ�ʯ����ȡǦ��Ӧѡ�����������е��Ȼ�ԭ����
��2���裺��������������������Ϊx��
Fe + H2SO4 == Fe SO4 + H2��
98 152
9.8t��20% x
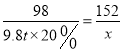
x=3.04t
�𣺿�������������������Ϊ3.04t��