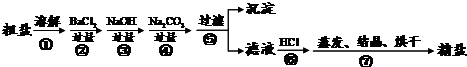
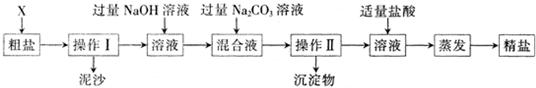
��Ŀ����
ͨ����ˮ��ɹ�ɵô��Σ����γ�NaCl�⣬������Na2SO4��MgCl2��CaCl2�Լ���ɳ�����ʣ�Ϊ����Ч�������ᴿ��ʵ��ĸ��������������£�
����������Ϣ�ش�
��1������ɲ�����֮ǰ��������������X�� ��y�������� ��
��2������ȥ��ҺA�е�Na2SO4��MgCl2��CaCl2�����ṩ���Լ���ѡ��a���������Լ������μ�˳������ Ϊ��ÿ�ζ���������NaOH��Һ�� �� ��������ĸ���ṩ���Լ��У�A��Na2CO3��Һ B��K2CO3��Һ C��BaCl2��Һ D��Ba��NO3��2��Һ��
��3������C�еijɷ���Mg��OH��2�� ���ѧʽ����
��4��ʵ���������õľ��ε��������ڴ������Ȼ��Ƶ�������ԭ���Ǵ����ᴿ���������µ�NaCl���ɣ����������в����µ�NaCl�ķ�Ӧ���� ����
��5������д����pH��ֽ�����ҺB���ȵľ��������ȡһС��pH��ֽ���ڽྻ�ĵ�ΰ��ϣ��� ���� �����ɶ�����Ӧ��ֵ��

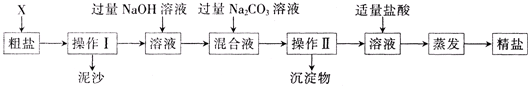
����������Ϣ�ش�
��1������ɲ�����֮ǰ��������������X��
��2������ȥ��ҺA�е�Na2SO4��MgCl2��CaCl2�����ṩ���Լ���ѡ��a���������Լ������μ�˳������ Ϊ��ÿ�ζ���������NaOH��Һ��
��3������C�еijɷ���Mg��OH��2��
��4��ʵ���������õľ��ε��������ڴ������Ȼ��Ƶ�������ԭ���Ǵ����ᴿ���������µ�NaCl���ɣ����������в����µ�NaCl�ķ�Ӧ����
��5������д����pH��ֽ�����ҺB���ȵľ��������ȡһС��pH��ֽ���ڽྻ�ĵ�ΰ��ϣ���
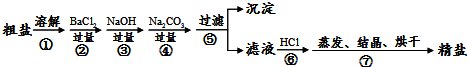
��������1�����ݴ��ε��ᴿ��Ҫ���������Һ���ܽ��У���������HCl����ȥ����NaOH��Na2CO3����
��2����ȥ�����к��е� Mg2+��Ca2+��SO42-���������ʵķ������������BaCl2��ȥ����������ӣ��������NaOH��ȥ��þ���ӣ����������Na2CO3��ȥ�������ӣ���
��3�����ݣ�2���Ľ�������жϣ������Ȼ�����̼���ƹ����ˣ�
��4�����ᴿ�������������Ȼ��ƣ�
��5����ϴ���IJ�����պȡ��������Һ��Ȼ�����һС��pH��ֽ�ϣ������ɫ���Ƚϣ�
��2����ȥ�����к��е� Mg2+��Ca2+��SO42-���������ʵķ������������BaCl2��ȥ����������ӣ��������NaOH��ȥ��þ���ӣ����������Na2CO3��ȥ�������ӣ���
��3�����ݣ�2���Ľ�������жϣ������Ȼ�����̼���ƹ����ˣ�
��4�����ᴿ�������������Ȼ��ƣ�
��5����ϴ���IJ�����պȡ��������Һ��Ȼ�����һС��pH��ֽ�ϣ������ɫ���Ƚϣ�
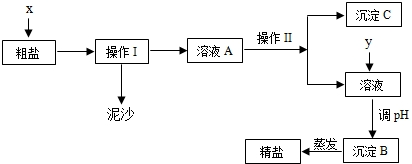
����⣺��1����ˮ��ɹ�õ��Ĵ����ǹ��壬Ҫ�����ᴿ����Ҫ�������Һ����������HCl����ȥ����NaOH��Na2CO3����
�ʴ�Ϊ��ˮ�����ᣮ
��2�����ݴ�����ȥ�����������ʣ����� Mg2+��Ca2+��SO42-�ķ�����
�ܽ⣺�������BaCl2��ȥ����������ӣ���SO42-+Ba2+=BaSO4����
�������NaOH��ȥ��þ���ӣ���Mg2++2OH-=Mg��OH��2����
�������Na2CO3��ȥ�������ӣ���Ca2++CO32-=CaCO3����
�ʴ�Ϊ��C��A��
��3�����ݣ�2����֪�����ijɷ��У�BaSO4��CaCO3��������Ȼ�����̼���ƹ���������̼������Һ���Ȼ�����Һ��Ӧ�����������Ȼ��ƺ�̼�ᱵ������
�ʴ�Ϊ��BaSO4��CaCO3��BaCO3��
��4���ڴ����ᴿ�Ĺ����У����ᴿ�������������Ȼ��ƣ�����ʵ�����þ��ε��������ڴ�����NaCl��������
���������в����µ�NaCl�ķ�Ӧ���У�MgCl2+2NaOH=Mg��OH��2��+2NaCl��Na2CO3+CaCl2�TCaCO3��+2NaCl��
BaCl2+Na2SO4�TBaSO4��+2NaCl��BaCl2+Na2CO3�TBaCO3��+2NaCl
�ʴ�Ϊ��4��
��5���ⶨpH��ķ�����ʹ��pH��ֽ���ⶨʱ���ò�����պȡ������Һ��������ֽ�ϣ�Ȼ���������ɫ�����գ���ɲ����Һ��pH�����Ǽ�����ҺpH����ȷ������
�ʴ�Ϊ��������պȡ������ҺB����pH��ֽ�ϣ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
�ʴ�Ϊ��ˮ�����ᣮ
��2�����ݴ�����ȥ�����������ʣ����� Mg2+��Ca2+��SO42-�ķ�����
�ܽ⣺�������BaCl2��ȥ����������ӣ���SO42-+Ba2+=BaSO4����
�������NaOH��ȥ��þ���ӣ���Mg2++2OH-=Mg��OH��2����
�������Na2CO3��ȥ�������ӣ���Ca2++CO32-=CaCO3����
�ʴ�Ϊ��C��A��
��3�����ݣ�2����֪�����ijɷ��У�BaSO4��CaCO3��������Ȼ�����̼���ƹ���������̼������Һ���Ȼ�����Һ��Ӧ�����������Ȼ��ƺ�̼�ᱵ������
�ʴ�Ϊ��BaSO4��CaCO3��BaCO3��
��4���ڴ����ᴿ�Ĺ����У����ᴿ�������������Ȼ��ƣ�����ʵ�����þ��ε��������ڴ�����NaCl��������
���������в����µ�NaCl�ķ�Ӧ���У�MgCl2+2NaOH=Mg��OH��2��+2NaCl��Na2CO3+CaCl2�TCaCO3��+2NaCl��
BaCl2+Na2SO4�TBaSO4��+2NaCl��BaCl2+Na2CO3�TBaCO3��+2NaCl
�ʴ�Ϊ��4��
��5���ⶨpH��ķ�����ʹ��pH��ֽ���ⶨʱ���ò�����պȡ������Һ��������ֽ�ϣ�Ȼ���������ɫ�����գ���ɲ����Һ��pH�����Ǽ�����ҺpH����ȷ������
�ʴ�Ϊ��������պȡ������ҺB����pH��ֽ�ϣ���ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
����������Ӷ���Ƕȶ��Ȼ��Ƶ��ᴿʵ�������ȫ�濼�죬�����ǿ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
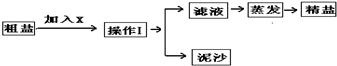
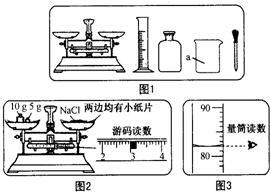 ijʵ��С������ͼ1��ʾ�������С�������������һ�����Ȼ�����Һ����ʵ�飺
ijʵ��С������ͼ1��ʾ�������С�������������һ�����Ȼ�����Һ����ʵ�飺