��Ŀ����
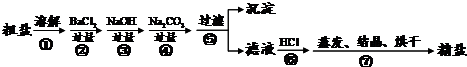
ͨ����ˮ��ɹ�ɵô��Σ����γ�NaCl�⣬������MgCl2��CaCl2�Լ���ɳ�����ʣ�Ϊ����Ч�������ᴿ��ʵ��ĸ���������������ͼ��ʾ��
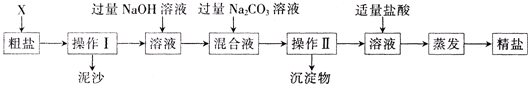
����������Ϣ�ش�
��1������ɲ�����֮ǰ����������X������
��2��������Ͳ������������

��3���������������������ʳ�ι���ɽ���Ϊ�������ٷɽ���������������ɲ�ȡ
��4����ʵ������м��������NaOH��Һ����ʵ��Ŀ����
��5���жϼ�������ᡰ�������ķ�����
��6��ʵ�����þ��ε�����

����������Ϣ�ش�
��1������ɲ�����֮ǰ����������X������
ˮ
ˮ
����2��������Ͳ������������
����
����
���ò�������Ҫ��Բ����ֽ�۵�����������ͼʾ�в��ó��ֵ�������D
D
������ţ���
��3���������������������ʳ�ι���ɽ���Ϊ�������ٷɽ���������������ɲ�ȡ
��Ъ���ȣ��������ƶ��ƾ��ƣ�
��Ъ���ȣ��������ƶ��ƾ��ƣ�
�ȴ�ʩ����4����ʵ������м��������NaOH��Һ����ʵ��Ŀ����
������Һ�е�þ����
������Һ�е�þ����
����5���жϼ�������ᡰ�������ķ�����
�μ������������ݷų�Ϊֹ
�μ������������ݷų�Ϊֹ
����6��ʵ�����þ��ε�����
����
����
������ڡ�����С�ڡ����ڡ���������NaCl��������ԭ�����ᴿ�Ĺ��������Ȼ�������
�ᴿ�Ĺ��������Ȼ�������
����������1�����ݴ��ε��ᴿ��Ҫ���������Һ���ܽ��У�
��2�������������Һ�ķ����ǹ��ˣ�
��3������������ע���������
��4���������ƿ��Խ�ˮ�е�þ����ת��Ϊ�������õ���ˮ��Ŀ�ģ�
��5��������̼���Ʒ�Ӧ�������ݲ�����
��6�����ᴿ�������������Ȼ��ƣ�
��2�������������Һ�ķ����ǹ��ˣ�
��3������������ע���������
��4���������ƿ��Խ�ˮ�е�þ����ת��Ϊ�������õ���ˮ��Ŀ�ģ�
��5��������̼���Ʒ�Ӧ�������ݲ�����
��6�����ᴿ�������������Ȼ��ƣ�
����⣺��1����ˮ��ɹ�õ��Ĵ����ǹ��壬Ҫ�����ᴿ����Ҫ�ȼ�ˮ�����Һ��
��2�������������Һ�ķ����ǹ��ˣ�I��II���Ƿ����������Ĺ��̣���ֽ�����ٶ���Ȼ��������з�϶����ѡD
��3���������������������ʳ�ι���ɽ���Ϊ�������ٷɽ������������裬���ɲ�ȡ��Ъ���ȣ��������ƶ��ƾ��ƣ��ķ�����
��4������������������ƣ����������ӿ��Ժ�þ����ת��Ϊ������þ��������þ���ӳ�ȥ��
��4���˲��dz��������̼���ƣ���������̼���Ʒ�Ӧ�������ݲ������������жϣ�
��5���ڴ����ᴿ�Ĺ����У����ᴿ�������������Ȼ��ƣ�����ʵ�����þ��ε��������ڴ�����NaCl��������
�ʴ�Ϊ����1��ˮ ��2������ D ��3����Ъ���ȣ��������ƶ��ƾ��ƻ������� ��4��������Һ�е�þ���� ��5���μ������������ݷų�Ϊֹ
��6������ �ᴿ�Ĺ��������Ȼ�������
��2�������������Һ�ķ����ǹ��ˣ�I��II���Ƿ����������Ĺ��̣���ֽ�����ٶ���Ȼ��������з�϶����ѡD
��3���������������������ʳ�ι���ɽ���Ϊ�������ٷɽ������������裬���ɲ�ȡ��Ъ���ȣ��������ƶ��ƾ��ƣ��ķ�����
��4������������������ƣ����������ӿ��Ժ�þ����ת��Ϊ������þ��������þ���ӳ�ȥ��
��4���˲��dz��������̼���ƣ���������̼���Ʒ�Ӧ�������ݲ������������жϣ�
��5���ڴ����ᴿ�Ĺ����У����ᴿ�������������Ȼ��ƣ�����ʵ�����þ��ε��������ڴ�����NaCl��������
�ʴ�Ϊ����1��ˮ ��2������ D ��3����Ъ���ȣ��������ƶ��ƾ��ƻ������� ��4��������Һ�е�þ���� ��5���μ������������ݷų�Ϊֹ
��6������ �ᴿ�Ĺ��������Ȼ�������
���������⿼�������ʵij��ӵ��й����⣬�ѶȱȽϴ��ر�ij�ȥʳ���еĶ������ʣ�Ҫ���ݳ��ӵ�ԭ������˼�����������Ż���˳�����ӳ��Ӽ���

��ϰ��ϵ�д�
�����Ŀ
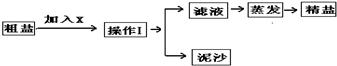


 ijʵ��С������ͼ1��ʾ�������С�������������һ�����Ȼ�����Һ����ʵ�飺
ijʵ��С������ͼ1��ʾ�������С�������������һ�����Ȼ�����Һ����ʵ�飺