��Ŀ����
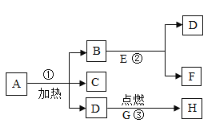
����Ŀ�����Ϻͻ�ѧ��ȤС���ͬѧ�����ɹ����˵�������Ȼ�������һ�֡����Ʒ���� ��̼���ơ��Ȼ��ƺ���ɳ����Ϊ�ⶨ������Ʒ����Ʒ�е���ɳ���μӷ�Ӧ��Ҳ������ˮ���� ̼���Ƶĺ��������õ�������һ��Ũ�ȵ��Ȼ�����Һ������������ʵ�飺
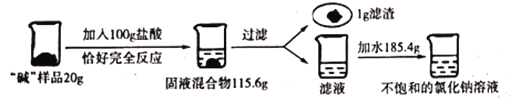
��ش��������⣺
��1��д��������Ӧ�Ļ�ѧ����ʽ____________��
��2��������֪�����г���ⷴӦ�������Ȼ��Ƶ�����(X)�ı���ʽ_______��
��3���á����Ʒ��̼���Ƶĺ���Ϊ_______��
��4�������յõ�����ҺΪ��������Һ����������Һ�����ʵ���������Ϊ______��
��5������36.5%��Ũ������������ʵ�����õ�ϡ���ᣬ��Ũ���������Ϊ________��
���𰸡�Na2CO3+2HCl=2NaCl+H2O+CO2�� ![]() 53�� 6.7�� 20g
53�� 6.7�� 20g
��������
���ɶ�����̼������=20g+100g-115.6g=4.4g��
�跴Ӧ�����Ȼ��Ƶ�����Ϊx����Ʒ��̼���Ƶ�����Ϊy���������������Ϊm��
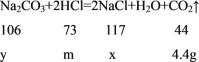
![]()
x=11.7g
y=10.6g
m=7.3g
��1��̼������ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2����
��2���г���ⷴӦ�������Ȼ��Ƶ�����(x)�ı���ʽΪ��![]() ��
��
��3���á����Ʒ��̼���Ƶĺ���Ϊ��![]() ��100��=53����
��100��=53����
��4����Ӧ����Һ���Ȼ��Ƶ�����=11.7g+��20g-10.6g-1g��=20.1g�������յõ�����ҺΪ��������Һ����������Һ�����ʵ���������Ϊ��![]() ��100��=6.7����
��100��=6.7����
��5������Ҫ36.5%��Ũ���������ΪA��36.5%��A=7.3��A=20g������Ũ���������Ϊ20g��

 �Ķ��쳵ϵ�д�
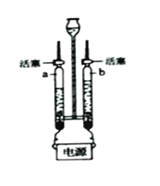
�Ķ��쳵ϵ�д�����Ŀ��С���Լ��еļ���������������Ũ�����Ȥ����ͨ���Ķ�˵�����˽�����������Ĺ���ԭ�����£���ѹʱ��װ�����������ڲ��ķ���ɸ���������еĵ�������ȡ��Ũ����������ѹʱ������ɸ���������ĵ����ŷţ����������У�����ɸ�������ģ�С������̨������������ȡ���ռ���һ�����壬����ʵ���Ҷ�������о���
ʵ��![]() ��С����ͨ������ʵ����֤���ռ��������Ƿ�Ϊ������������
��С����ͨ������ʵ����֤���ռ��������Ƿ�Ϊ������������
��ʵ���¼��
ʵ�鲽�� | ʵ������ | ��ѧ����ʽ |
ȡһֻ | ľ̿ȼ�յø��� | ��ѧ����ʽ |
���У���ѧ����ʽ![]() ��________��
��________��
��ʵ����ۣ��ռ��������Ǵ�����������
��ʵ�鷴˼����ʦָ������ʵ�鲢����֤��С��������������һ���Ǵ�������������ʵ�鲻��֤�����ռ�����Ϊ����������ԭ����________��
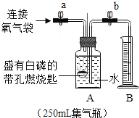
ʵ��![]() ���ⶨС�����������������ĺ�����
���ⶨС�����������������ĺ�����
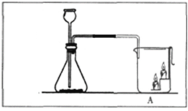
ͬѧ������ʦ��ָ�����������ͼ��ʾװ�ã�����ʵ�飬���ظ���Σ�
��ʵ���¼��
ʵ����� | ��Ҫʵ������ | ʵ����ۼ����� | |
�� | ���װ�õ������� | / | װ�õ����������� |
�� | ����ҩƷ����װ�����ӹ̶� | / | / |
�� | ��ֹˮ�� |
| �ռ� |
�� | �����۹���ȼ���� | ȼ�գ������������� | / |
�� | ������Ϩ����ȴ�����£� ���в��� | ���� | С���ռ������岻�Ǵ��������� |
��������![]() ��________������
��________������![]() ��________��
��________��
�����ݼ�¼��
ʵ����� |
|
|
|
|
|
|
|
|
|
|
|
�����ݴ�������֪�����ϱ������У���![]() ������ƫ��ϴ��������������ݴ���ʱӦɾȥ��С�������������������������Ϊ________
������ƫ��ϴ��������������ݴ���ʱӦɾȥ��С�������������������������Ϊ________![]() ��
��
��ʵ�鷴˼��
��1����̨�����������������Ĺ��̷����ı仯����________��������仯����ѧ�仯������
��2�����µ�![]() �����ݲ����ϴ����Ŀ���ԭ����________��
�����ݲ����ϴ����Ŀ���ԭ����________��