��Ŀ����
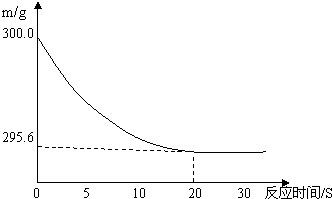
��6�֣���ʢ��22.3 g Na2CO3��NaCl����������ձ��м���216.1 gϡ����ǡ�÷�Ӧ,��Ӧ�����þ�����������ձ���ͬҩƷ������(m)�뷴Ӧʱ��(t)�Ĺ�ϵ����ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300 g���ش��������⣺
(1)������������ϡ����ǡ����ȫ��Ӧʱ������ʱ��ԼΪ S��
(2)��ȫ��Ӧ����������̼�������� g��
(3)��Ӧ�õ������µIJ�������Һ��������Һ�����ʵ���������Ϊ���٣�
��6�֣�
��1��20��1�֣��� ��2��4.4 g��1�֣���
��3���⣺��μӷ�Ӧ��̼���Ƶ�����Ϊx����Ӧ���ɵ��Ȼ��Ƶ�����Ϊy
Na2CO3 + 2HCl =" 2NaCl" + CO2�� + H2O
106 117 44
x y 4.4g ����1��
106/44=x/4.4g ,117/44=11.7/4.4g
��ã�x="10.6" g��y="11.7" g�� ����1��
�ձ��ﲻ������Һ������������Ϊ�� 11.7 g + (22.3 g��10.6 g) =" 23.4" g��
�ձ��ﲻ������Һ������Ϊ�� 22.3 g+216.1 g��4.4 g =" 234" g������1��
���ò�������Һ����������������23.4 g/ 234 g ��100%=10%������1��
����

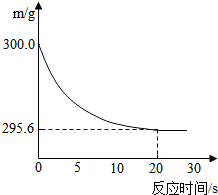 ��ʢ��22.3 g Na2CO3��NaCl����������ձ��м���216.1 gϡ����ǡ�÷�Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ����ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300 g���ش��������⣺
��ʢ��22.3 g Na2CO3��NaCl����������ձ��м���216.1 gϡ����ǡ�÷�Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ����ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300 g���ش��������⣺