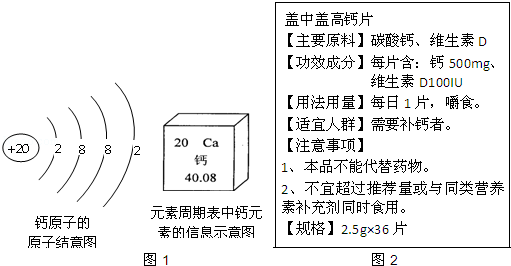
��Ŀ����
С��ͬѧ���ڿ����з���һ��ʱ��ġ�ͭ��������˿����������ͭ��Һ�Ƴ�����ͼ���ĵijɷ�ʱ�з�����ȡ��64.1�˹�����Ʒ����10%���������ܽ⣬��Һ����ɫ�������������ʣ�����������10%�����������仯��ϵ��������ͼ��
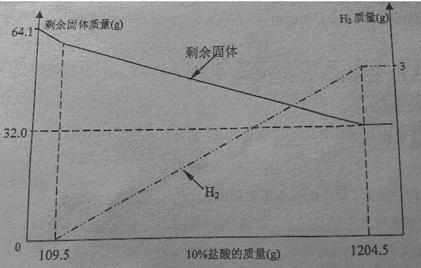
���ͼ�����ݷ�����
��1����ͼ��֪��CuԪ�ص�����_____g��64.1g��ͭ������Cu��Al��_____���ѧʽ��
��2�����ϻ�ѧ����ʽ����á�ͭ������AlԪ�ص�����������
��3��ֻ֪����ͭ��������m1������10%��������������m2��,Ҳ�������ͭ��������Ԫ�����������������ʽΪ_______________(��m1��m2��ʾ���ɲ�����)

���ͼ�����ݷ�����
��1����ͼ��֪��CuԪ�ص�����_____g��64.1g��ͭ������Cu��Al��_____���ѧʽ��
��2�����ϻ�ѧ����ʽ����á�ͭ������AlԪ�ص�����������
��3��ֻ֪����ͭ��������m1������10%��������������m2��,Ҳ�������ͭ��������Ԫ�����������������ʽΪ_______________(��m1��m2��ʾ���ɲ�����)
��1��32.0 Al2O3 (2)64.3% ��3��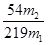 ��100%
��100%
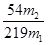 ��100%
��100%(1)ͭ�������ᷴӦ��Al�������ᷴӦ������������ˮ����ʣ����������Ϊͭ��������Al��CuSO4��Һ��Ӧ������Cu��Al2(SO4)3��
��2���������Ʒ��Al������Ϊx��
2Al + 6HCl = 2AlCl3 + 3H2��
54 6
x 3g
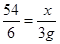
x=27g
Al2O3��ϡ���ᷴӦʱ���������������Ϊ109.5g��10%=10.95g
��Al2O3������Ϊy��
Al2O3 + 6HCl = 2AlCl3 + 3H2O��
219
y 10.95
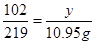
y=5.1g
Al2O3��Al������Ϊ5.1g��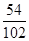 ��100%=2.7g
��100%=2.7g
��Ʒ��Al������Ϊ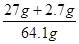 ��100%=46.3%
��100%=46.3%
�𣺸á�ͭ������AlԪ�ص���������Ϊ46.3%��
��2���������Ʒ��Al������Ϊx��
2Al + 6HCl = 2AlCl3 + 3H2��
54 6
x 3g
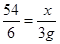
x=27g
Al2O3��ϡ���ᷴӦʱ���������������Ϊ109.5g��10%=10.95g
��Al2O3������Ϊy��
Al2O3 + 6HCl = 2AlCl3 + 3H2O��
219
y 10.95
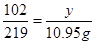
y=5.1g
Al2O3��Al������Ϊ5.1g��
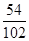 ��100%=2.7g
��100%=2.7g��Ʒ��Al������Ϊ
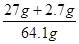 ��100%=46.3%
��100%=46.3%�𣺸á�ͭ������AlԪ�ص���������Ϊ46.3%��

��ϰ��ϵ�д�
�����Ŀ
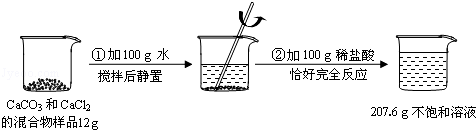
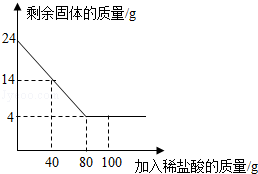

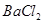 �Ĺ�������49.3g������167g����ˮ����ȫ�ܽ����û����Һ����μ���142g��
�Ĺ�������49.3g������167g����ˮ����ȫ�ܽ����û����Һ����μ���142g��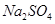 ��Һ��ǡ�÷�Ӧ����
��Һ��ǡ�÷�Ӧ����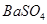 ����������Ϊ23.3g������ʾ��BaCl2+Na2SO4=BaSO4��+2NaCl��������ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�
����������Ϊ23.3g������ʾ��BaCl2+Na2SO4=BaSO4��+2NaCl��������ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�