��Ŀ����
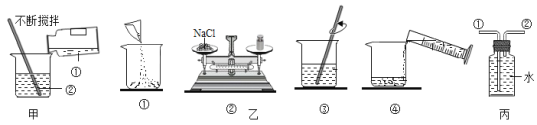
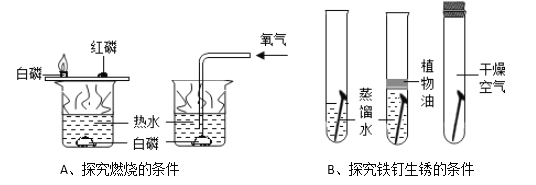
����Ŀ����ͼ�Ǽ��ֹ������ʵ��ܽ�����ߡ�
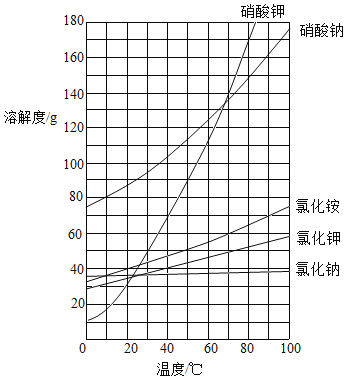
��1��80��ʱ��KCl��NH4Cl��NaNO3���ܽ���ɴ�С����Ϊ______��
��2��20��ʱ��50gˮ�м���20g NaCl����ֽ�����γ�______��Һ����������������������������
��3��60��ʱ���ֱ���ͬ������NaCl��NH4Cl��KNO3������Һ������20�棬������������������______��
��4��60��ʱ������KNO3��Һ������������������߲��ᳬ��______����ȷ��0.1%����
���𰸡�NaNO3��NH4Cl��KCl ���� KNO3 52.4%
��������
��1�����ܽ�����߿�֪��80��ʱ��KCl��NH4Cl��NaNO3���ܽ���ɴ�С����Ϊ��NaNO3��NH4Cl��KCl���ʴ�Ϊ��NaNO3��NH4Cl��KCl��
��2��20��ʱ��50gˮ�м���20gNaCl����ֽ�����γɱ�����Һ�������Թܵײ��й����������ʴ�Ϊ�����ͣ�
��3��60��ʱ���ֱ���ͬ������NaCl��NH4Cl��KNO3������Һ������20����������������������KNO3����Ϊ����ص��ܽ�����¶�Ӱ��仯��ʴ�Ϊ��KNO3��
��4��60��ʱ������KNO3��Һ������������������߲��ᳬ���� ![]() ���ʴ�Ϊ��52.4%��
���ʴ�Ϊ��52.4%��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�����Ŀ����ͼ���������ʵ���У����ǣ�C12H22O11����ڣ��������,������ɵ�̿���ų��д̼�����ζ�����塣

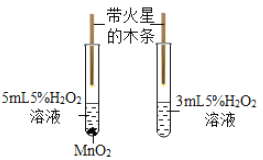
��������⣩�ı��ǵ����ࡢŨ����������ˮ�ĵ������¶��Ƿ��Ӱ��ʵ��Ч����?
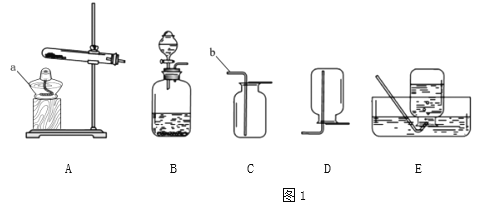
������ʵ�飩�ֱ�ȡ5g�Ƿ۽���ʵ�飬����ǰ2min�������ʵ��Ч���������֣�
��¼���£�
��1ʵ���¼
ʵ�� | �� | Ũ�������/mL | ˮ�ĵ��� | �¶�/�� | Ч���÷� |
1 | ���� | 4 | 5 | 22 | 65 |
2 | ���� | 5 | 10 | 30 | 81 |
3 | ���� | 6 | 15 | 50 | 93 |
4 | ���� | 4 | 10 | 50 | 91 |
5 | ���� | 5 | 15 | 22 | 88 |
6 | ���� | a | 5 | 30 | 75 |
7 | ������ | 4 | 15 | 30 | 0 |
8 | ������ | 5 | 5 | 50 | 0 |
9 | ������ | 6 | 10 | 22 | 0 |
���������ݣ�������1������Ч���÷־�ֵ��������£�
��2Ч���÷־�ֵ
���� | �� | Ũ�������/mL | ˮ�ĵ��� | �¶�/�� | ||||||||
���� | ���� | ������ | 4 | 5 | 6 | 5 | 10 | 15 | 22 | 30 | 50 | |
��ֵ | 79.7 | 84.7 | 0 | 52.0 | 56.3 | 56.0 | 46.7 | 57.3 | 60.3 | 51.0 | 52.0 | 61.3 |
���ݾ�ֵ�ƶ����ʵ�鷽������ֵԽ�ߣ�Ч��Խ�á�
����������ۣ�
��1�����DZ�ɺ�ɫ��̿��������_____________������������������ѧ�����仯��
��2���Ʋ�ų������庬SO2����Ԫ���غ�Ƕ�˵�����ɣ�________________________________��
��3��ʵ��6�У�aΪ_____________mL��
��4�����ݱ�2�ƶϣ����������ʵ�����ѡ��5g���ǡ�5mLŨ���ᡢ_____��ˮ��50��
����˼�����ۣ�
��5����2�У�22���Ӧ�ľ�ֵΪ51.0�������ֵ�����ݵ�3�����ݷֱ���_____________________��
��6���ۺϷ�����ʹʵ��3��Ч���÷ָ���ʵ��4��������________________________________��
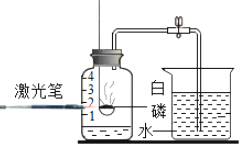
����Ŀ����Ƭ�ı�ǩ��ͼ��ʾ����֪�˸�Ƭ�ɷ���ֻ��̼����к��и�Ԫ�أ��˱�ǩ�еĺ������Ƿ���ȷ��
��1��______�����ȷ��������Ϊ�ⶨ�京������С��ÿ��ȡ10Ƭ��Ƭ�����ѳ����ĺ�����ϡ������ձ��У���ַ�Ӧ���ٳ�ȡ�ձ���ʣ�������������С����������ʵ���������£�
���ʵ����� | ��1�� | ��2�� | ��3�� | ƽ��ֵ |
��Ӧǰ���ձ�+ϡ���� | 22g | 22g | 22g | 22g |
10Ƭ��Ƭ | 8g | 8g | 8g | 8g |
��Ӧ���ձ�+ϡ���� | 26.7g | 26.5g | 26.9g | 26.7g |
��2��ÿƬ��Ƭ�к�̼���������____g
��3��ͨ��С����ʵ�飬�㽨�鳧������ı�ǩ��______��