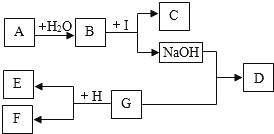
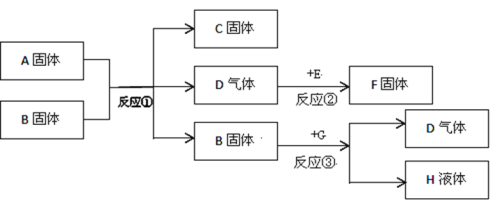
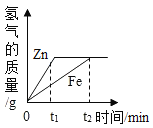
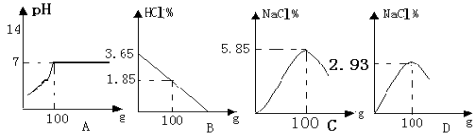
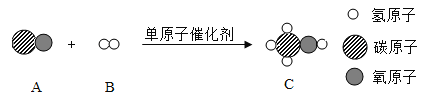
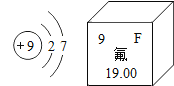
��Ŀ����
����Ŀ�������Σ���ܴ�������������γ�һ����������;����
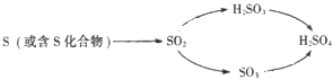
��1��������ˮ�������ԣ���Ҫԭ������ ����
��2��pH��5.6�Ľ�ˮ��Ϊ���꣬����ʹ�����ữ��ʩ������ �ɸ����������ԣ�
��3��SO2ͨ���ˮ��I2��ˮ��Һ�����������ᣨH2SO4��������ᣨHI�������ǿ����ô�ԭ�����ⶨ������SO2�ĺ�����SO2�ı仯���̿ɱ�ʾΪ��SO2![]() H2SO4��д���÷�Ӧ�Ļ�ѧ����ʽ���� ����
H2SO4��д���÷�Ӧ�Ļ�ѧ����ʽ���� ����
���𰸡���1��������̼��ˮ��Ӧ����̼�ᣬ̼�����Һ�����ԣ�2����ʯ�ң�3��SO2+2H2O+I2�TH2SO4+2HI
��������
�����������1����������ˮ��Ϊ����̼��������ԣ�������̼��ˮ��Ӧ����̼�ᣬ̼�����Һ�����ԣ���2�������Σ���ܴ��������ữ����ʴ������ȣ�ũҵ�ϳ�����ʯ��������������������3��SO2ͨ���ˮ��I2��ˮ��Һ�����������ᣨH2SO4��������ᣨHI������ѧ����ʽΪ��SO2+2H2O+I2�TH2SO4+2HI��

��ϰ��ϵ�д�
�����Ŀ