��Ŀ����
������1����ѧ�����ɼ����Ŀ�״ʯ��ʯ��Ʒ��ˮ��ϴ�����ɣ��Ƶ���Ʒ����Ϊ25.0g���ð�ס�������ͬѧ��������25.0gʯ��ʯ��Ʒ�ֱ����������ʵ�顣��������Ʒ���������ʲ��μӷ�Ӧ��������ˮ���Ȼ����ݳ���
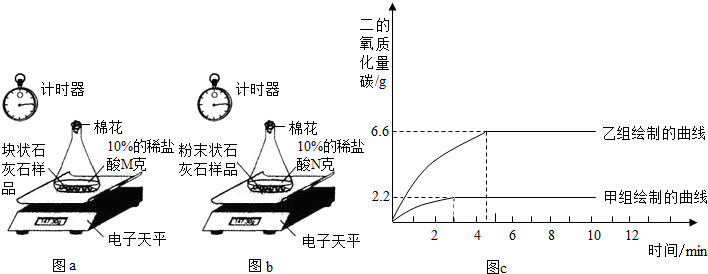
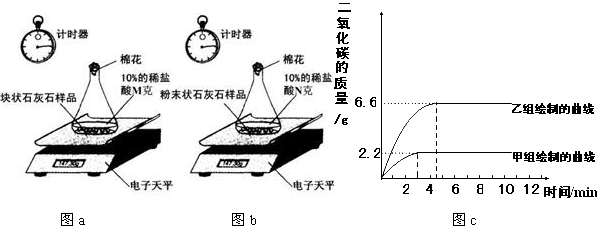
��ʵ����̡����飺ȡһ�������Ŀ�״ʯ��ʯ��Ʒ����ƿ�ڣ����������10%��ϡ����M g���ⶨ��Ӧ��������ƿ�е�ҩƷ�����仯������ͼa��
���飺��ʣ��Ŀ�״ʯ��ʯ��Ʒ����ɷ�ĩ״��Ȼ��ȫ��������һ��ƿ�ڣ�����10%��ϡ����N g���ⶨ��Ӧ��������ƿ��ҩƷ�������仯������ͼb��
����ͬѧ�����ݴ����õ��ͷų�������̼�������뷴Ӧʱ��Ĺ�ϵ��ͼc��
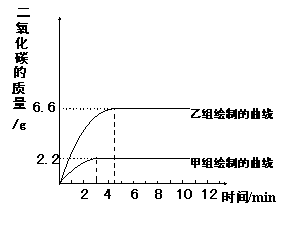 |
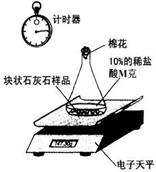
ͼa ͼb ͼc
���������ۡ�
��1���ס�������ͬѧ��ʵ���У� ��ʵ�����ĵ�ʱ����̡�
��2������ʵ�������ĵ�ϡ���������ȼ�: ��=__________��
��3����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��1���ף�1�֣� ��2��1:3 ��1�֣�
��3�� �۽⣺��ÿ����Ʒ��CaCO3������Ϊx
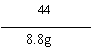
CaCO3+2HCl==CaCl2+H2O+CO2�� ��1�֣�
100 73 44
x 2.2g+6.6g
![]()
![]() =
= 
![]() x��20g ��1�֣�
x��20g ��1�֣�
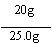
![]() ��100����80��0�� ��1�֣�
��100����80��0�� ��1�֣�
����Ʒ��CaCO3��������Ϊ80��0��
(ע��������������У���Ч���ֵı�������Ӳ��Ҫ����������)