��Ŀ����
�����⣺�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��٣�
���𰸡������������������ͭ�Ļ����Һ�μ�����������Һ�����ᡢ����ͭ�������������Ʒ�����Ӧ����������Ĵ�����������������ͭ��������������ͭ�����������ᷴӦ����ܲ���������ͭ���������Լ�¼�����У�����50.0g����������Һʱ��������������Ϊ0�����ڼ�������������Һ200.0g�Ժ�����������ٱ仯��˵������ͭҲ����ȫ��Ӧ�������ɳ��������ֵΪ9.8g��
��������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ���ɳ���������ͭ�������ɼ�������Һ������ͭ��������
���������ἰ����ͭ��Ӧ���������Ƶ�������������������Һ���������������ıȿɼ���μ�����������Һ��������
����⣺��1���ɼ�¼���ݱ���֪��������ͭ��ȫ��Ӧ��������ɫ����9.8g��
����Һ������ͭ������Ϊx
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
x 9.8g
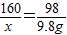
x=16g
��2���������ᷴӦ��NaOH������Ϊy����CuSO4��Ӧ��NaOH������Ϊz��
H2SO4+2NaOH=Na2SO4+2H2O
98 80
9.8g y

y=8.0g
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
80 98
z 9.8g
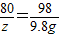
z=8g
�ʲμӷ�Ӧ��NaOH��Һ��������Ϊ =160g
=160g
�����������вμӷ�Ӧ������������Һ����������160�ˣ�
�������ڽ���漰��Ӧ����������ʱ��Ҫע��������㡢�۵���������壬���Ϊ��Ӧ��ʼ�������۵����Ǹ÷�Ӧǡ����ȫ��Ӧ��ѧ��Ӧ��Ϥ���û�ѧ����ʽ�����˼·��ʽ��������ǡ�÷�Ӧ��Ԫ���غ�ͷ������������
��������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ���ɳ���������ͭ�������ɼ�������Һ������ͭ��������
���������ἰ����ͭ��Ӧ���������Ƶ�������������������Һ���������������ıȿɼ���μ�����������Һ��������
����⣺��1���ɼ�¼���ݱ���֪��������ͭ��ȫ��Ӧ��������ɫ����9.8g��
����Һ������ͭ������Ϊx
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
x 9.8g
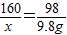
x=16g
��2���������ᷴӦ��NaOH������Ϊy����CuSO4��Ӧ��NaOH������Ϊz��
H2SO4+2NaOH=Na2SO4+2H2O
98 80
9.8g y

y=8.0g
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
80 98
z 9.8g
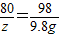
z=8g
�ʲμӷ�Ӧ��NaOH��Һ��������Ϊ
 =160g
=160g�����������вμӷ�Ӧ������������Һ����������160�ˣ�
�������ڽ���漰��Ӧ����������ʱ��Ҫע��������㡢�۵���������壬���Ϊ��Ӧ��ʼ�������۵����Ǹ÷�Ӧǡ����ȫ��Ӧ��ѧ��Ӧ��Ϥ���û�ѧ����ʽ�����˼·��ʽ��������ǡ�÷�Ӧ��Ԫ���غ�ͷ������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�ձ���װ��һ�������������ͭ�Ļ����Һ����֪����Һ�к�H2SO4������Ϊ9.8g��ijͬѧΪ�ⶨ�û����Һ������ͭ�����������ձ�������10%��NaOH��Һ���õ�������������¼���£�
��1���õ�������������Ϊ g���û����Һ������ͭ������Ϊ g��
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߣ����������
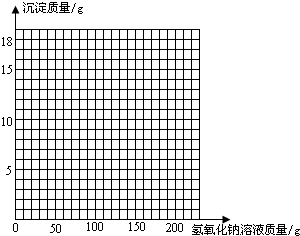
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
��2���μӷ�Ӧ��NaOH��Һ���������Ƕ��ٿˣ���Ҫ��д��������̣�
��3�������ڸû����Һ�м���NaOH��Һ���������ɳ��������仯��ϵ�����ߣ����������

ijʵ��С���ʵ�����ƶ�����̼��Ӧ���ʵ�Ӱ�������Լ���Ӧ��IJ��������̽����
��̽��Ӱ�췴Ӧ���ʵ����أ�ʵ�����õ��Լ��ͷ�Ӧ�������£�
ʵ��С����Ƶ�ʵ�鷽���������±����������Ϣ�����ѱ����ȱ��д������
��Է�Ӧ���Һ�ɷֵ�̽����
Ϊ������Һ������Ĵ��ڣ�ʵ��С��ͬѧ�����������������м��飬���ж���Щ�����Ƿ������д�����ɣ�
ʵ��֤�����÷�Һ�к����ᣮ
��Ӧ����Һ���Ȼ��Ƶ������ⶨ��
�ձ���װ��һ�����ķ�Ӧ��Һ����̽����֪�÷�ҺΪ�Ȼ��ƺ�����Ļ����Һ�����к��Ȼ���3.65g��ʵ��С��Ϊ�˲ⶨ�����Һ���Ȼ��Ƶ����������ձ�����μ���10.6%��̼������Һ���õ�������������¼���£�
��1���õ�������������Ϊ g���û��Һ���Ȼ��Ƶ�����Ϊ g��
��2���μӷ�Ӧ��̼������Һ���������Ƕ��ٿˣ�д��������̣�
��3��д���ڸû����Һ�м���̼������Һ�����������ɳ��������仯��ϵ�����ߣ�
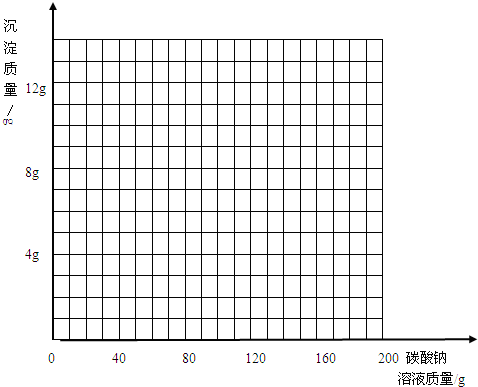
��̽��Ӱ�췴Ӧ���ʵ����أ�ʵ�����õ��Լ��ͷ�Ӧ�������£�

ʵ��С����Ƶ�ʵ�鷽���������±����������Ϣ�����ѱ����ȱ��д������
| ʵ���� | �¶� | ����ʯ | ����Ũ�� | ʵ��̽��Ŀ�� |
| �� | 20�� | �ֿ��� | 5% | ��ʵ��ٺ͢�̽��Ũ�ȶԷ�Ӧ������Ӱ�죮 ��ʵ��ں� ��ʵ��ٺ͢�̽������ʯ��ϸ�Է�Ӧ������Ӱ�죮 |
| �� | 20�� | �ֿ��� | ||
| �� | ϸ���� | 5% | ||
| �� | 40�� | 10% |
Ϊ������Һ������Ĵ��ڣ�ʵ��С��ͬѧ�����������������м��飬���ж���Щ�����Ƿ������д�����ɣ�
| ���� | ���Ƿ���� | ˵�����ɣ�д��ѧ����ʽ�� |
| ����Һ��pH�Ƿ�С��7 | ���� | �Ȼ�����������Һ������������ԣ� |
| �μ���������Һ�۲��Ƿ��а�ɫ�������� | ||
| �����۹۲��Ƿ������� |
��Ӧ����Һ���Ȼ��Ƶ������ⶨ��
�ձ���װ��һ�����ķ�Ӧ��Һ����̽����֪�÷�ҺΪ�Ȼ��ƺ�����Ļ����Һ�����к��Ȼ���3.65g��ʵ��С��Ϊ�˲ⶨ�����Һ���Ȼ��Ƶ����������ձ�����μ���10.6%��̼������Һ���õ�������������¼���£�
| ����̼������Һ������/g | 40.0 | 80.0 | 120.0 | 160.0 | 200.0 |
| ���ɳ���������/g | 0.0 | 3.0 | 7.0 | 10.0 | 10.0 |
��2���μӷ�Ӧ��̼������Һ���������Ƕ��ٿˣ�д��������̣�
��3��д���ڸû����Һ�м���̼������Һ�����������ɳ��������仯��ϵ�����ߣ�
