��Ŀ����
ij��ѧ��ȤС���ͬѧ�� �����ᡢ���ᱵ���������ơ�̼���ơ���������֮��ķ�Ӧ�����˶��ԺͶ������о������ݸ��ֽⷴӦ�����������������ж�����������Һ����֮���ܷ��� ����Ӧ�����������塢�������ɵĻ�ѧ����ʽΪ�������д��һ����
��1�� ����2�� ��
��ʵ��һ��֤������������Һ��ϡ�����Ϻ�ȷʵ�����˻�ѧ��Ӧ
��ͬѧ�����з���������ʵ�飺 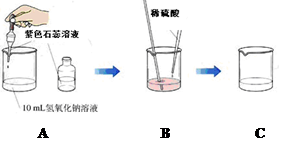
��ش�
��A��ʵ���У�������ɫʯ����Һ��Ŀ���� ���� ����
Bװ���з����Ļ�ѧ��Ӧ����ʽΪ ����
|
��ͬѧ��ȡ38.2g�������ƺ�̼���ƵĻ����Һ���ձ��У�
��������εμ���������Ϊ15.3%��ϡ���ᣬͬʱ��¼ʵ�����ݣ�
��ü���ϡ��������������������������ϵ����ͼ��ʾ��
�ٵ�����16gϡ����ʱ����Һ�е�����
Ϊ ��д��ѧʽ��
����������ϡ����ǡ����ȫ��Ӧʱ����
��Һ��������������������д��������̣����������0.1%��
��ʵ������ʵ��������ֻ�������Һ��Ϻ�ijɷ�
��ͬѧ���������̽�����ʵ�飺
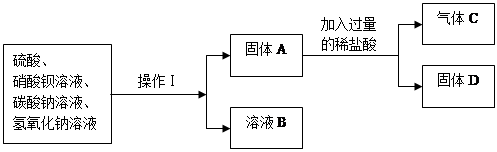
������������� ������D�Ļ�ѧʽ�� ��
����ҺB�е����̪������ɫ����B��һ��û�� ���ӣ�һ������ ���ӡ�
4 �� (1)Na2CO3+ H2SO4 =Na2SO4+H2O+CO2�� ��
(2) H2SO4+Ba(NO3)2 =BaSO4��+ 2HNO3 �� Ba(NO3)2+Na2CO3=BaCO3 ��+ 2NaNO3
��ʵ��һ��֤������������Һ��ϡ�����Ƿ�����Ӧ �� 2NaOH + H2SO4 = Na2SO4 + 2H2O
��ʵ�������NaOH��Na2CO3��Na2SO4
�ڣ�4�֣�������һ��
�⣺���������ϡ����ǡ����ȫ��Ӧʱ����ϡ���������ʵ�����Ϊ
64g��15.3% �� 9.8g
���������ϡ����ǡ����ȫ��Ӧʱ������Һ�е���������Ϊ��
9.8g����142��98��=14.2g
���������ϡ����ǡ����ȫ��Ӧʱ������Һ�е�������������Ϊ��
����������
�⣺��:��̼���Ʒ�Ӧ��ϡ��������Ϊx�����������Ƶ�����Ϊy
Na2CO3+ H2SO4 = Na2SO4+H2O+CO2��
98 142 44
15.3%��x y 2.2g
98
x="32g" �� y=7.1g
���������Ʒ�Ӧ����������Ϊ��64g-32g����15.3%=4.9g
��:���������Ʒ�Ӧ���ɵ������Ƶ�����Ϊz
2NaOH + H2SO4 = Na2SO4 + 2H2O
98 142
4.9g z
Z=7.1g
���������ϡ����ǡ����ȫ��Ӧʱ������Һ�е�������������Ϊ��
��ʵ���������ˣ�BaSO4�� OH-��Na+��NO3-��
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�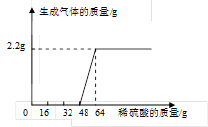
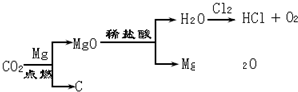
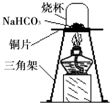 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����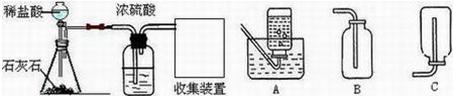
 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������