��Ŀ����
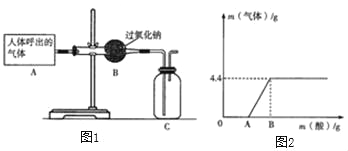
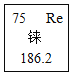
����Ŀ��ijͬѧΪ�˲ⶨCaCl2��CaCO3����������CaCO3��������������������ʵ�飺��20g������������ձ��ڣ���ȡ100g���ᣬƽ���ֳ���ݣ����μ��룬�������ʵ�����ݣ�
ʵ����� | һ | �� | �� | �� | �� |
����������Һ��������g�� | 20 | 20 | 20 | 20 | 20 |
�����������������g�� | 1.1 | m | 3.3 | 4.4 | 4.4 |
�ʣ���1������m��ֵΪ g��
��2��ԭ���������е�CaCO3��������Ϊ ��
��3��ǡ����ȫ��Ӧʱ������Һ������������������Ҫ��д��������̣�
���𰸡�2.2��50%��22.1%
��������
��1����һ�μ���20g������Һ�õ�����1.1g��������ʵ�����20g������Һ�������������ˣ�˵���ڶ�����̼��ƻ�û����ȫ��Ӧ�����Եڶ��μ����20g������Һ��ȫ��Ӧ����1.1g���壬����m��ֵΪ2.2�����2.2��
��2������ͼ����Ϣ��֪���������ɶ�����̼���������Ϊ4.4g��
����Ʒ��̼��Ƶ���������Ϊx��
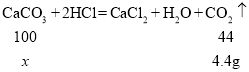
![]()
x=10g
ԭ���������е�CaCO3��������=![]() =50%
=50%
���50%��
��3����ǡ����ȫ��Ӧʱ������Һ������CaCl2������Ϊy��
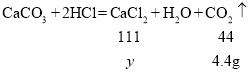
![]()
y=11.1g
ǡ����ȫ��Ӧʱ������Һ���ʵ���������=![]() ��22.1%
��22.1%
��ǡ����ȫ��Ӧʱ������Һ��������������Ϊ22.1%��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
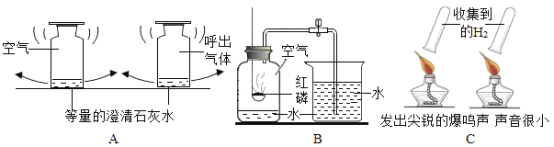
Сѧ��ĩ���Ծ�ϵ�д�����Ŀ��ijͬѧ�ڰ���ʵ��Ա������ѧ�Լ�ʱ������һƿ��ǩ��ȱ����ɫ��Һ����ͼ����ʾ������ʵ��Ա������֪ԭƿ��Һ�е����ʿ�����NaCl��NaOH��Na2CO3��NaHCO3�е�һ�֣�Ϊȷ����Һ�е����ʣ�����Ʋ�����������̽�����
�����ϲ��ģ������������ʵ������Ϣ���£�
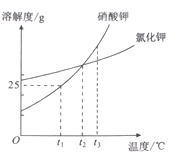
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�����µ��ܽ��/g | 36 | 109 | 21.5 | 9.6 |
������ϡ��Һ��pH | 7 | 13 | 11 | 9 |
��ش��������⣺
��ʵ��̽��1��
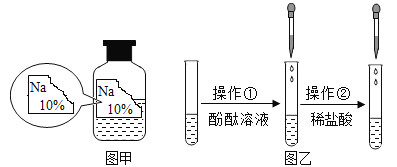
����ͼ����ʾ���ڲ����ٺ��ȷ�����ʲ���NaCl����ʵ������Ӧ��______���ڽ��в�����ʱ����ɫ��ζ����������ɴ��ֿ��ų���������__________��
��̽�����ۣ������������ʵ������Ϣ������Ϊ����Һ�е����ʿ������������������е�______������ж�������__________��������̽����������ȷ�ģ������ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________��
���������ɣ���ͬѧ��Ϊ���Ϸ��������ܣ���Ҫ��һ��ʵ��ȷ���������ֽ���������̽����
��ʵ��̽��2��������ٺ��Թ��е���Һ�еμӹ���CaC12��Һ�����ԣ�����ַ�Ӧ���Թ�����Һ��ɫ���䣬���а�ɫ����������
���ó����ۣ�ͨ��ʵ��̽��2��ȷ��ԭƿ��Һ�е�����Ӧ����_________��
����˼��չ����ɸ���Һ��������ʵ�������ԭ����______________���û�ѧ����ʽ��ʾ����
����Ŀ��ijУѧϰС���ͬѧΪ�˲ⶨijʯ��ʯ��̼��Ƶ���������������ȡ��ʯ��ʯ��Ʒ10g�����ձ��У��ٰ�80gϡ������Ĵμ��룬ʵ����������������±�����֪ʯ��ʯ��Ʒ�к��е����ʼȲ�����ˮ��Ҳ����ϡ���ᷴӦ����
ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
����ϡ���������/g | 20 | 20 | 20 | 20 |
ʣ����������/g | 7 | 4 | 2.6 | m |
�����������ݣ�����������⣺
��1������m����ֵΪ_____g��
��2����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ_____��
��3�����㷴Ӧ����ϡ����������������_____��