��Ŀ����
����Ŀ��ʵ���ǿ�ѧ̽������Ҫ������������һ������ʶ���������������IJⶨ��
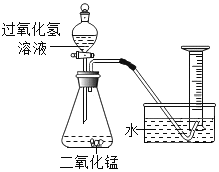
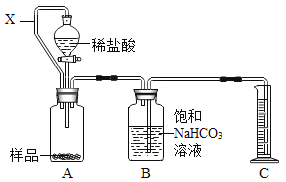
��ʵ��عˣ���ȤС���ͬѧ���ÿα��е�ʵ�鷽��������ͼ���ⶨ�����������ĺ�����������������������������С��1/5.�������̽����

��������⣩�������ԭ����ʲô����θĽ��α��ϵ�ʵ��װ�ã�
���������ϣ����ס�����һЩ�������±���
��ɫ��״̬ | �ܵ��� | �Ż���� | �ܶ�/��g/cm3�� | |
���� | ����ɫ���� | 590 | 240 | 2.34 |
���� | ��ɫ���ɫ���� | 44.1 | 40 | 1.82 |
����������裩
��ͬѧ�������ǵ�����ԭ���п��������������������ˮ��Ӱ��ʵ���ȷ�ԡ�
��ͬѧ��������ƿ�ڲ��������л���������
��1���㻹�������IJ�����_________________��
��ʵ���뽻����
��2������ʦ��ָ���£�С��ͬѧ�Կα��ϵ�ʵ��װ�ý�������ͼ��ʾ�ĸĽ�������������·������ͬѧ���Ӧ�Ѻ���Ϊ���ף�������____________________��
��̽���뷴˼��
��3������øĽ���ʵ��װ�ý���ʵ�飬��ע������ȴ�����ڰ�����ʧ�������ⷢ��ע�����ڱڸ��Ż�ɫ���壬�û�ɫ�������Ҫ�ɷֿ�����_________________��
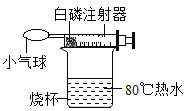
��4��Ϊ����֤��ͬѧ�IJ��룬����ʦ��ָ���£���ȤС���ͬѧ�����ô������Ľ�ʵ��װ�ã��ֱ��ù����ĺ��Ͱ����ж���ʵ�飬���ø��ܼ���ʵ�ȼ�ס�
���ò�������Ũ�ȵĴ�������÷�Ӧ��װ��������Ũ�ȷֱ��ǣ�����Ϊ8.7%�Ͱ���Ϊ10%���Ӷ�֤����ͬѧ�IJ���_____________��������ȷ����������������
����ͼ�ǰ���ȼ�չ����е�ѹǿ�仯���ƣ��Է����ش�ͼ��AB�α仯��ԭ����_______________��

���𰸡�װ��©������û����ȴ�����¾ʹ�ֹˮ�У����������ɣ� �����Ż��Ƚϵ� ���� ��ȷ ����ȼ�շ��ȣ����¼���ƿ�е��������ͣ���ѹ����
��������
��1�������Ƿ�������ϣ����ж����������Ĺؼ����ס�����ͬѧ���Ǵ��ڲ�˵���ܷ�װ���ڻ��в��������������һ�ֿ��ܿɴ��ⲿ���ͣ�װ��©��ƿ����������û����ȴ�����¾ʹ�ֹˮ�У�ƿ�ڵ���ѹ�ϸߣ�
��2����ȼ���Ż���ﵽ�Ż�㣬�ձ�����ˮΪ80�棬�����Ż��Ϊ240�棬����Ϊ40�棬����Ҫ�ڴ�װ�õ�ȼ��ȼ���ѡ���Ż��͵İ��ף���ͬѧ���Ӧ�Ѻ���Ϊ���ף������ǰ����Ż��Ⱥ��ͣ����ڵ�ȼ��
��3������Ϊ����ɫ���壬�������������ʣ��ж�ʣ�µ�Ϊδ��Ӧ�İ��ף�
��4��װ���ڼ���������Ũ�ȣ�˵�������������ڣ�������ͬѧ�IJ�������ȷ�ģ�������ȼ�չ��̷ų��������ȣ����ܱ����������ڷ�Ӧ���ڽ��У���ʱ�ų�����ʹ���ڲ�ѹǿ����������������С��ѹǿ�����Գ��ֳ�ѹǿ��������ߡ�

����Ŀ�����������������������й㷺Ӧ�á�ʵ��С��Թ��������ijЩ���ʽ����о���
���ȶ���
��1����ͼ��ʾ����ʵ�飬��������ֽ�Ļ�ѧ����ʽΪ______������3.2gO2ʱ�ֽ�Ĺ������������Ϊ______g��
��2��������ˮ���ռ�O2��ԭ����______��
��3��̽���¶ȶԹ�������ֽ����ʵ�Ӱ��
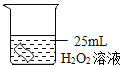
ͬѧ�ǽ��������µ�ʵ�飬ʵ���������±���
ʵ����� | �� | �� | �� |
H2O2��Һ��Ũ��% | 30 | 30 | 30 |
H2O2��Һ�����/mL | 6 | 6 | 6 |
�¶�/�� | 20 | 35 | 55 |
MnO2������/g | 0 | 0 | 0 |
�ռ�O2�����/mL | 0 | 1.9 | 7.8 |
��Ӧʱ�� | 40min | 40min | 40min |
�ɴ˵ó��Ľ�����______��
��ʴ��
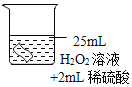
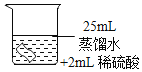
���������ϣ�H2O2��Һ�и�ʴ�ԡ�
������ʵ�飩
��ͭƬ�ֱ������3����Һ�н���ʵ�飬���±���
��� | �� | �� | �� |
ʵ�� |
|
|
|
һ��ʱ�������� | �����Ա仯 | ��Һ��������������ϸС���� | �����Ա仯 |
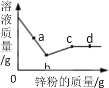
����������ۣ�
��4��ʵ��ٵ�������______��
��5��ͭƬ����ʴ�ķ�Ӧ���£���ȫ�÷�Ӧ�Ļ�ѧ����ʽ��Cu+H2O2+H2SO4=CuSO4+______��
����˼������
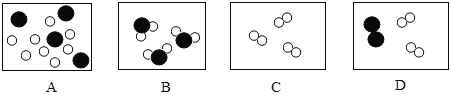
��6��ijͬѧ�����ʵ����У���������5���ķ�Ӧ�⡣��������һ����Ӧ������ϸС���ݲ������÷�Ӧ�ķ�Ӧ��Ϊ______��