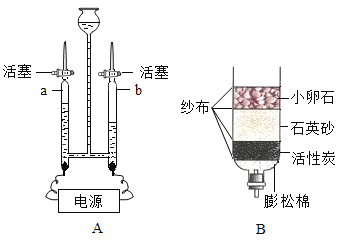
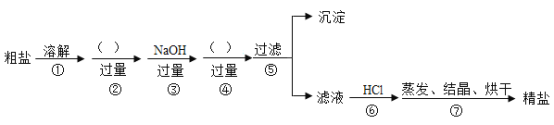
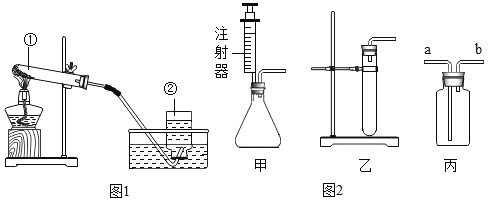
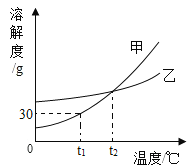
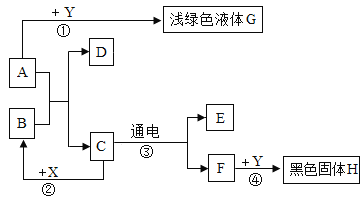
��Ŀ����
����Ŀ��ijˮ��Һ�к������������е������֣�K+��Cl-��Ca2+��H+��CO32-��SO42-����ȡ������Һ�и�100ml���ֱ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����BaCl2��Һ�ó�����6.63g,����������ϴ�ӡ������������Ϊ4.66g;��������Һ�м���AgNO3��Һ����Һ���ɡ���������ʵ�飬�ش��������⡣
��1��д���ڣ�2���������ɳ����ķ�Ӧ����ʽ��__________��
��2�����ݵڣ�1����2����ʵ������ж�ԭ��Һ��_______�������϶����������϶������������������� CO32-��SO42-��
��3��ԭ��Һ��CO32-��SO42-�ĸ�����Ϊ________
��4��ԭ��Һ�п϶�û�е����ӣ�__________��
���𰸡�BaCl2+K2SO4=BaSO4��+2KCl��BaCl2+K2CO3=BaCO3��+2KCl �϶��� 1��2 Ca2+��H+
��������
����AgNO3��Һ�г���������˵����Һ�п��ܺ��������ӡ�̼������ӻ���������ӣ��ڶ�����Һ�����Ȼ�����Һ���ó�����6.63g������������ϴ�ӡ������������Ϊ4.66g�������������٣�˵���п����ᷴӦ���ܽ��̼���γ�����˵�������Ȼ�����������ֳ����������Һ��һ������̼������Ӻ���������ӣ���Ϊþ���ӡ�����������̼������ӽ�ϳɳ����������Һ��һ������þ���Ӻͱ����ӣ�̼������Ӻ������ӽ�����ɶ�����̼��ˮ�������棬��Һ��һ��û�������ӣ������Ӻ�̼����������ɳ����������棬��Һ��һ��û�и����ӣ���Һ���������ӵĵ���غ㣬��Һ�е�������ֻ�м����ӣ����һ������������K+������ȷ���Ƿ��������ӡ�������Һ�к���CO32-��SO42-����Ca2+��H+��CO32-�����棬����û��Ca2+��H+���ָ��������غ㣬һ����K+��
��1��д���ڣ�2���������ɳ����ķ�Ӧ����ʽ��BaCl2+K2SO4=BaSO4��+2KCl��BaCl2+K2CO3=BaCO3��+2KCl��
��2�����ݵڣ�1����2����ʵ������ж�ԭ��Һ����CO32-��SO42-����������Cl-��
��3��̼�ᱵ�������ᣬ���ᱵ���ܣ�̼�ᱵ��̼�ᱵ��6.63g���������ᱵ4.66g����̼�ᱵ������Ϊ1.97g��ԭ��Һ��CO32-��SO42-�ĸ�����Ϊ1.67g
![]() ��
��![]() =1��2��
=1��2��
��4��ԭ��Һ�п϶�û�е�����Ca2+��H+��

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�