��Ŀ����
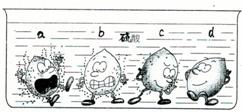
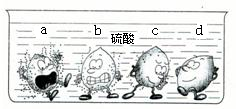
ѧ�������Ļ��˳���Ժ�����ͬѧ����һϵ��֪ʶ�������о�����1������ͼ�е�ʵ�������жϣ�ϡ������a��b��c��d���ֽ����У�λ�������Ľ�����______��
��2��������Ni��Mn�ֱ�����������ֽ���X��Y��Z������Һ�У���Ӧ�Ľ����������������������ұ���ʾ�������ǵĽ��������ǿ������˳��Ϊ��______��
| X����Һ | Y����Һ | Z����Һ | |
| Ni | �� | �� | �� |
| Mn | �� | �� | �� |
B��Z Ni Y Mn X
C��X Mn Y Ni Z
D��X Mn Ni Y Z
��3���������ϣ������ڻ��˳�����λ��Խ�����仯����Խ���ȶ�����������������Խ�ױ���ԭ������д�������ý�����������ȡ�����Ļ�ѧ����ʽ��
�ٽ�����������ȷֽ⣺______ 2Hg+O2��
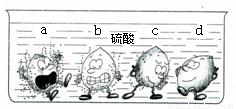
���𰸡���������1���۲�ͼ�з���ϡ���е����ֽ����������ݵĶ��٣��жϽ����Ļ�ԣ�
��2������ʵ�����жϽ������˳��
��3�����������ķ�Ӧ���ɣ�ѡ����Ϥ�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ��
��4��������̼�������ж����壬�������������Һ��Ӧ����ѡ������������Һ�������գ��Է�ֹ�����Ⱦ��
��5�����ʵ�飬���ͭ������ͭ��ת��������ͭ�����ʣ��ɽ��У�Cu��CuO��CuSO4��ת����
��6�����ݷ�Ӧ�Ļ�ѧ����ʽ������ͭ���������������������̼�����������������������������ԭϡ������Һ������������
����⣺��1����������ų���������Խ�����Ļ��Խǿ����֮��
ͼ�У�����d���������ݣ�˵���ý������ܺ�ϡ���ᷴӦ���ڽ����˳����оʹ���H֮��
��ѡD��
��2����ʵ������֪��Ni�Ļ��С��X��Y������Z��Mn�Ļ��С��X������Y��Z��
�����ֽ����У�X�Ļ����ǿ��Z�Ļ����������ǿ������˳��ΪX��Ni��Y��Ni��Z��
��ѡC��
��3���ٴ��෴Ӧ���࣬��ɫ��ĩ���������ȣ��ֽ�ɽ��������ͷų�������
�ʴ�2HgO 2Hg+O2����
2Hg+O2����
��̼�ڸ������������ʽϻ��ã��ܻ�ԭһЩ����������������ͭ�ȣ����ɺ�ɫͭͬʱ�ų����������̼��
��ѡC+2CuO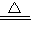 2Cu+CO2�������������𰸾��ɣ���
2Cu+CO2�������������𰸾��ɣ���
��4��������̼Ϊ�ǽ���������������Һ������������Һ��Ӧ�������������ƺ�ˮ���ж����������̼�����գ����ٶԿ�����ɵ���Ⱦ��
�ʴ��������ƣ�NaOH������ֹ���ɵĶ���������Ⱦ������
��5�����ý���ͭ�ڼ���ʱ�������������ɺ�ɫ����ͭ����ͭ���м��ȣ��ѵõ��ĺ�ɫ����ͭ����ϡ���ᣬ����ͭ�����ᷴӦ��Ϳ��Եõ�����ͭ��
�ʴ�2Cu+O2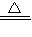 2CuO��CuO+H2SO4��ϡ���TCuSO4+H2O��
2CuO��CuO+H2SO4��ϡ���TCuSO4+H2O��
��6���裺����SO2������ΪX������H2SO4������ΪY
Cu+2H2SO4��Ũ���TCuSO4+SO2��+2H2O
64 196 64
8g Y X
64��64=12.8g��X����֮�� X=12.8g
64��196=12.8g��Y����֮�� Y=39.2g
������Һ�����ʵ���������Ϊ��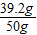 ×100%=78.4%
×100%=78.4%
������SO2������Ϊ12.8g��������Һ�����ʵ���������Ϊ78.4%��
���������ǿ�Ľ����ܰѻ�����Ľ�����������Һ���û���������֮���ܰѽ�����������Һ���û�������˵���������ǿ��
��2������ʵ�����жϽ������˳��
��3�����������ķ�Ӧ���ɣ�ѡ����Ϥ�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ��
��4��������̼�������ж����壬�������������Һ��Ӧ����ѡ������������Һ�������գ��Է�ֹ�����Ⱦ��
��5�����ʵ�飬���ͭ������ͭ��ת��������ͭ�����ʣ��ɽ��У�Cu��CuO��CuSO4��ת����
��6�����ݷ�Ӧ�Ļ�ѧ����ʽ������ͭ���������������������̼�����������������������������ԭϡ������Һ������������
����⣺��1����������ų���������Խ�����Ļ��Խǿ����֮��
ͼ�У�����d���������ݣ�˵���ý������ܺ�ϡ���ᷴӦ���ڽ����˳����оʹ���H֮��
��ѡD��
��2����ʵ������֪��Ni�Ļ��С��X��Y������Z��Mn�Ļ��С��X������Y��Z��
�����ֽ����У�X�Ļ����ǿ��Z�Ļ����������ǿ������˳��ΪX��Ni��Y��Ni��Z��
��ѡC��
��3���ٴ��෴Ӧ���࣬��ɫ��ĩ���������ȣ��ֽ�ɽ��������ͷų�������
�ʴ�2HgO
 2Hg+O2����
2Hg+O2������̼�ڸ������������ʽϻ��ã��ܻ�ԭһЩ����������������ͭ�ȣ����ɺ�ɫͭͬʱ�ų����������̼��
��ѡC+2CuO
 2Cu+CO2�������������𰸾��ɣ���
2Cu+CO2�������������𰸾��ɣ�����4��������̼Ϊ�ǽ���������������Һ������������Һ��Ӧ�������������ƺ�ˮ���ж����������̼�����գ����ٶԿ�����ɵ���Ⱦ��
�ʴ��������ƣ�NaOH������ֹ���ɵĶ���������Ⱦ������
��5�����ý���ͭ�ڼ���ʱ�������������ɺ�ɫ����ͭ����ͭ���м��ȣ��ѵõ��ĺ�ɫ����ͭ����ϡ���ᣬ����ͭ�����ᷴӦ��Ϳ��Եõ�����ͭ��
�ʴ�2Cu+O2
 2CuO��CuO+H2SO4��ϡ���TCuSO4+H2O��
2CuO��CuO+H2SO4��ϡ���TCuSO4+H2O����6���裺����SO2������ΪX������H2SO4������ΪY
Cu+2H2SO4��Ũ���TCuSO4+SO2��+2H2O
64 196 64
8g Y X
64��64=12.8g��X����֮�� X=12.8g
64��196=12.8g��Y����֮�� Y=39.2g
������Һ�����ʵ���������Ϊ��
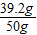 ×100%=78.4%
×100%=78.4%������SO2������Ϊ12.8g��������Һ�����ʵ���������Ϊ78.4%��
���������ǿ�Ľ����ܰѻ�����Ľ�����������Һ���û���������֮���ܰѽ�����������Һ���û�������˵���������ǿ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ѧ�������Ļ��˳���Ժ�����ͬѧ����һϵ��֪ʶ�������о���
ѧ�������Ļ��˳���Ժ�����ͬѧ����һϵ��֪ʶ�������о�����1������ͼ�е�ʵ�������жϣ�ϡ������a��b��c��d���ֽ����У�λ�������Ľ�����
��2��������Ni��Mn�ֱ�����������ֽ���X��Y��Z������Һ�У���Ӧ�Ľ����������������������ұ���ʾ�������ǵĽ��������ǿ������˳��Ϊ��
| X����Һ | Y����Һ | Z����Һ | |
| Ni | �� | �� | �� |
| Mn | �� | �� | �� |
B��Z Ni Y Mn X
C��X Mn Y Ni Z
D��X Mn Ni Y Z
��3���������ϣ������ڻ��˳�����λ��Խ�����仯����Խ���ȶ�����������������Խ�ױ���ԭ������д�������ý�����������ȡ�����Ļ�ѧ����ʽ��
�ٽ�����������ȷֽ⣺
����̼��ԭ���������
��4���������ϣ��ڽ������˳���λ�������Ľ���ͭ�ڳ�������Ȼ������ϡ���ᡢ
ϡ���ᷴӦ����������Ũ�����ڼ���ʱ������Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ��
Cu+2H2SO4��Ũ��?CuSO4+SO2��+2H2O
��Ӧ���ɵ�SO2��һ����ɫ��������ˮ���д̼�����ζ�����壮���ø÷�����ȡ����ͭ��Ӧ��
��5������������ѧ֪ʶ���һ����ͭ��ϡ����Ϊ��Ҫԭ����ȡ����ͭ�ķ��������û�ѧ����ʽ��ʾ��
��6����12.8gͭ�м���40gŨ������Һ��ǡ����ȫ��Ӧ����������SO2������������ʽ���ϣ��ͷ�Ӧǰ������Һ�����ʵ�����������
 ѧ�������Ļ��˳���Ժ�������һϵ��֪ʶ�������о���
ѧ�������Ļ��˳���Ժ�������һϵ��֪ʶ�������о��� ѧ�������Ļ��˳���Ժ�������һϵ��֪ʶ�������о���
ѧ�������Ļ��˳���Ժ�������һϵ��֪ʶ�������о��� 4Al2O3+9Fe��
4Al2O3+9Fe��