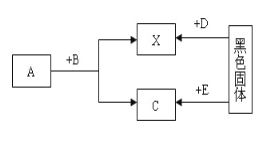
��Ŀ����
����Ŀ��H2�ڼ��ȵ������¿��Ի�ԭCuO(![]() )��ij��ȤС��ͬѧ��һ���������˽:ͭ��CuO��Cu2O(��ɫ)���ֳ����������Cu��Cu2O��Ϊ��ɫ�������Dz²⣬��H2��ԭCuO���õĺ�ɫ�����п��ܺ���Cu2O��
)��ij��ȤС��ͬѧ��һ���������˽:ͭ��CuO��Cu2O(��ɫ)���ֳ����������Cu��Cu2O��Ϊ��ɫ�������Dz²⣬��H2��ԭCuO���õĺ�ɫ�����п��ܺ���Cu2O��
(1)��С��ͬѧȷ��ȡ��8.0g��ɫCuO��ĩ������Թ��У�����ͨ��������H2���ڼ��������½��л�ԭ���������ֻ��Cu��������ӦΪ ��
(2)��С����H2��ԭCuOʱ��������8.0g��ɫCuO��ĩȫ��ת��ɺ�ɫʱ��ֹͣ���ȣ���ȴ��Ƶù�������Ϊ6.8g���˺�ɫ��������Ԫ�ص�����Ϊ ���Դ��Ƶ�Cu2O������Ϊ ��
(3)��(2)�����õ�6.8g��������ձ��У�����48.0g����ϡ����(Cu2O��ϡ����ķ�ӦΪ:Cu2O + 2HCl�TCuCl2+Cu+H2O )����ֽ��裬���к�ɫ�����⣬��������Һ������̡��Լ������ɫ��Һ��CuCl2����������(��ȷ��0.01 % )��
���𰸡���1��6.4g����2��0.4g����3��6.75%
��������(1)������������ֻ��Cu��������ӦΪxg
CuO��H2 ![]() Cu��H2O
Cu��H2O
80 64
8.0g x
![]() ��
��![]() ,x��6.4g��
,x��6.4g��
(2) ��ɫ��������Ԫ�ص�����Ϊ��8.0g��![]() ��100�����v8.0g��6.8g�w��0.4g.
��100�����v8.0g��6.8g�w��0.4g.
�Դ��Ƶ�Cu2O������Ϊ��![]() ��
��![]() ,x��3.6g.
,x��3.6g.
(3) �������ɫ��Һ��CuCl2������Ϊxg,����ͭ����Ϊyg
Cu2O + 2HCl�TCuCl2+Cu+H2O
144 135 64
3.6g x y
![]() ��
��![]() , x��3.4g
, x��3.4g
![]() ��
��![]() ,y��1.6g
,y��1.6g
��ɫ��Һ��CuCl2������������![]() ��100����6.75��.
��100����6.75��.
�㾦�ñ�����Ҫ������ݻ�ѧ����ʽ���м��㡣

����Ŀ����1��Ϊ֤���кͷ�Ӧ�Ƿ��ȷ�Ӧ��ijС���������ͼ��ʾ��ʵ�������
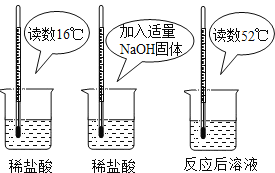
������ͼʵ�飬��ͬѧ��Ϊ��NaOH��ϡ���ᷢ�����кͷ�Ӧ�Ƿ��ȷ�Ӧ����ͬѧ��Ϊ����ͬѧ�ó�������۵����ݲ���ѧ��������_____________________________����ͬѧ��Ϊ��ͨ�����ʵ����ܵó�һ���ձ�Ľ��ۡ������پٳ�һ������кͷ�Ӧ��ʵ����д����Ӧ�Ļ�ѧ����ʽ_____________________________��
��2��Ϊ̽��Ӱ���кͷ�Ӧ�ų��������ٵ����أ������ֽ���������ʵ�飺�ڱ��ΪA��B��C��D��E����ֻ�ձ��и�װ��36.5g������������Ϊ5%��10%��15%��20%��25%�����ᣬ����������ֻ�ձ��зֱ����40g20%������������Һ�����������¶ȣ����ݼ�¼���£�
�ձ���� | A | B | C | D | E |
����������������� | 5% | 10% | 15% | 20% | 25% |
��Ӧ����Һ�¶ȣ��棩 | 24�� | 34�� | 46�� | 54�� | 54�� |
��ʵ����ۡ�Ӱ���кͷ�Ӧ�ų��������ٵ�������____________________________��
��������˼����Ӧ���ձ�����ҺpH��С����______�����ձ���ţ���
��Ҫʹ40g20%������������Һǡ����ȫ�кͣ�����Ҫ10%��������Һ���ٿˣ������ݻ�ѧ����ʽ��ʽ���㣩__________