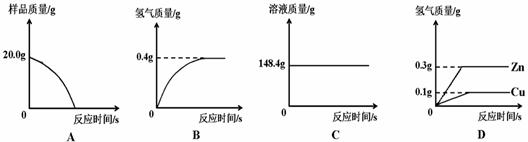
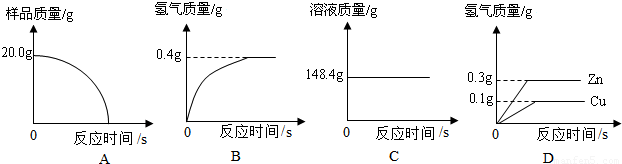
��Ŀ����
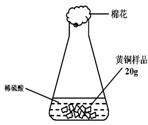
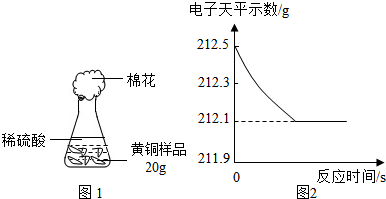
��ͭ��ͭп�Ͻ�����������������Ҫ��;��ij�о���ѧϰС���ͬѧ��Ϊ�ⶨij��Ʒ���е�п�����������Ƿ���Ϲ���Ĺ涨��32��38%�����������ͼ1��ʾ��ʵ��װ�ý��м�⣮
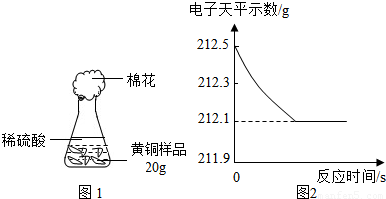
��1����������Բ���ʼʵ��ʱ���ȴ�Һ©���ϿڵIJ���������������������һ�����ϡ����Ҳ����˳��������ƿ���������������ҵ��������֢�ᣬ����ͼ2װ�ý��У����е���a��������
��2���������ĺϽ�����Ϊ10g����ַ�Ӧ����ȡ���������Ϊ1000mL��ͨ���������Ʒ�Ƿ�ϸ���֪�������ܶ�Ϊ0.089g/L��
��3������һ��С���ͬѧ��������ƽ������Ҳ���Ե�����˲ⶨ�������ȳ�ȡm1g�Ͻ������ձ�������Ϊm2g���У�����������ϡ���ᣨ����Ϊm3g�����ٲ��������ˣ��Ƶ��ձ���ʣ������������Ϊm4g������m1+m2+m3��-m4=0.2g��Ҫ��֤�Ͻ���Ʒ�е�п��ȫ��Ӧ��m1����Ϊ���ٿˣ���д�����������̣�
��˼������2���еĸ����������䣬�����ã�3���ķ������вⶨ�Ƿ���У�������

��1����������Բ���ʼʵ��ʱ���ȴ�Һ©���ϿڵIJ���������������������һ�����ϡ����Ҳ����˳��������ƿ���������������ҵ��������֢�ᣬ����ͼ2װ�ý��У����е���a��������
ƽ����ƿ���Һ©���ڵ�ѹǿ
ƽ����ƿ���Һ©���ڵ�ѹǿ
��ʹ����˳������
ʹ����˳������
����2���������ĺϽ�����Ϊ10g����ַ�Ӧ����ȡ���������Ϊ1000mL��ͨ���������Ʒ�Ƿ�ϸ���֪�������ܶ�Ϊ0.089g/L��
��3������һ��С���ͬѧ��������ƽ������Ҳ���Ե�����˲ⶨ�������ȳ�ȡm1g�Ͻ������ձ�������Ϊm2g���У�����������ϡ���ᣨ����Ϊm3g�����ٲ��������ˣ��Ƶ��ձ���ʣ������������Ϊm4g������m1+m2+m3��-m4=0.2g��Ҫ��֤�Ͻ���Ʒ�е�п��ȫ��Ӧ��m1����Ϊ���ٿˣ���д�����������̣�
��˼������2���еĸ����������䣬�����ã�3���ķ������вⶨ�Ƿ���У�������
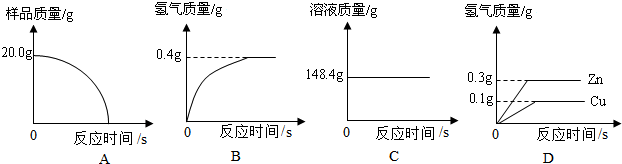
�����У����ٵ�����Ϊ0.089g������������ƽ������
�����У����ٵ�����Ϊ0.089g������������ƽ������
����������1������װ���ص㼰����ѹǿ����Һ��Ӱ�������
��2�������������������������������������п���������Ӷ�����Ͻ���п������������ȷ���Ƿ�ϸ�
��3������������������п����������������������������0.1g������ƽ��������
��2�������������������������������������п���������Ӷ�����Ͻ���п������������ȷ���Ƿ�ϸ�
��3������������������п����������������������������0.1g������ƽ��������
����⣺��1��п��ϡ���ᷴӦ����������ʹ��ƿ����ѹ������ƿ�ڵ�ѹǿ���ڴ���ѹ������aʹ��ƿ�ڵ���������Һ©���Ϸ���ʹ��Һ©���Ϸ���ѹǿ����ϡ����˳�����£�
�ʴ�Ϊ��ƽ����ƿ���Һ©���ڵ�ѹǿ��ʹϡ����˳�����£�
��2������Ʒ��п��������X
Zn+H2SO4�TZnSO4+H2��
65 2
X 1L��0.089g/L=0.089g
=
��X=2.89g
����Ʒ��п�����������ǣ�
��100%=28.9%��32%
�𣺸���Ʒ���ϸ�
��3��������0.2g��������Ҫп��������Y
Zn+H2SO4�TZnSO4+H2��
65 2
Y 0.2g
=
Y=6.5g
m1=
=17.1g
��m1����Ϊ17.1��
[��˼]������2���еĸ����������䣬�����ã�3���ķ������вⶨ���÷������У���Ϊ���ٵ�����Ϊ0.089g����ƽ�ܾ�ȷ��0.1g��������������ƽ������
�ʴ�Ϊ�������У����ٵ�����Ϊ0.089g������������ƽ������
�ʴ�Ϊ��ƽ����ƿ���Һ©���ڵ�ѹǿ��ʹϡ����˳�����£�
��2������Ʒ��п��������X
Zn+H2SO4�TZnSO4+H2��
65 2
X 1L��0.089g/L=0.089g
| 65 |
| X |
| 2 |
| 0.089g |
��X=2.89g
����Ʒ��п�����������ǣ�
| 2.89g |
| 10g |
�𣺸���Ʒ���ϸ�
��3��������0.2g��������Ҫп��������Y
Zn+H2SO4�TZnSO4+H2��
65 2
Y 0.2g
| 65 |
| Y |
| 2 |
| 0.2g |
Y=6.5g
m1=
| 6.5g |
| 38% |
��m1����Ϊ17.1��
[��˼]������2���еĸ����������䣬�����ã�3���ķ������вⶨ���÷������У���Ϊ���ٵ�����Ϊ0.089g����ƽ�ܾ�ȷ��0.1g��������������ƽ������
�ʴ�Ϊ�������У����ٵ�����Ϊ0.089g������������ƽ������
������������Ҫ���������÷���ʽ�ļ��㼰��ʵ��װ�õķ������ܺܺõĿ���ѧ��Ӧ����ѧ֪ʶ�����ͽ���������������һ���Ѷȣ�

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ


 ������������Ʒ����Ҫ���ý����������õ����Ե��ǣ�___ ____��
������������Ʒ����Ҫ���ý����������õ����Ե��ǣ�___ ____��