��Ŀ����
��2011?������һģ���������������������������Ź㷺��Ӧ�ã���1������������Ʒ����Ҫ���ý����������õ����Ե��ǣ�______��

��2����ҵ�����У��и�����ʱ������ͭ��Һ�������ϻ��߿����º�ɫ��ӡ�����йط�Ӧ�Ļ�ѧ����ʽΪ______��
��3����Ҫ�Ƚ������̡�ͭ�Ľ������ǿ������ѡ���ҩƷ��������ͭ�����⣬����Ҫ______��
��4���о���ѧϰС��Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ��õ�����ƽ�ֱ�Ƶ���ƿ����������Ϊ44.1g����ȡ��ͭ��Ʒ20.0g������ƿ�м������Ʒ������ϡ�����ƿ������������ͼ1��ʾ����������ƽ���������ݻ��ͼ2��
������������ݣ��ش��������⣺
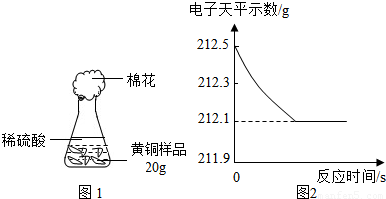
�����ĸ�ͬѧ�Ӷ�Ƕȴ������ݣ��������ݴ�����ͼ������ȷ����______��
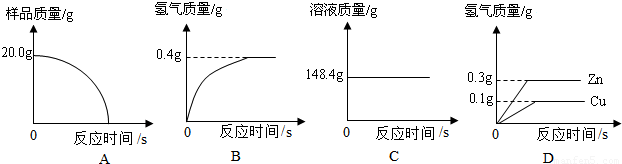
�����Լ��㣺����Ʒ��ͭ��������������ǡ�÷�Ӧʱ������Һ�����ʵ�����������
���𰸡���������1�������������õĵ����ԣ������������ߣ�
��2����������ͭ��Ӧ����������������ͭ��
��3������ͭ���ã�Ҫ��֤�̵Ļ����ԣ���ѡ���ҩƷ��������ͭ�����⣬����Ҫ�̵�����Һ��
��4������ͼ����Ϣ�ͻ�ѧ����ʽ���Խ�����ط���ļ��㣮
����⣺��1�����������������˽����ĵ����ԣ����A��
��2����������ͭ��Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4�TCu+FeSO4��
��3������ͭ���ã�Ҫ��֤�̵Ļ����ԣ���ѡ���ҩƷ��������ͭ�����⣬����Ҫ�̵�����Һ�����MnSO4��Һ���̵�����Һ��
��4��������ͼ�����ݿ�֪����������������Ϊ0.4g�����B��
�����Լ��㣺
�ٽ⣺����Ʒ��п������ΪX�����ɵ�����п������ΪY��
Zn+H2SO4=ZnSO4+H2��
65 161 2
X Y 0.4g
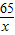 =
= ��
��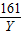 =
=
X=13.0g��Y=32.2g��
M��Cu��=20g-13g=7g��
��Ʒ��ͭ����������Ϊ�� ×100%=35%��
×100%=35%��
������п��Һ����������Ϊ�� ×100%=20%��
×100%=20%��
����Ʒ��ͭ����������Ϊ35%������п��Һ����������Ϊ20%��
������������Ҫ�������ͻ�ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�
��2����������ͭ��Ӧ����������������ͭ��
��3������ͭ���ã�Ҫ��֤�̵Ļ����ԣ���ѡ���ҩƷ��������ͭ�����⣬����Ҫ�̵�����Һ��
��4������ͼ����Ϣ�ͻ�ѧ����ʽ���Խ�����ط���ļ��㣮
����⣺��1�����������������˽����ĵ����ԣ����A��
��2����������ͭ��Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4�TCu+FeSO4��
��3������ͭ���ã�Ҫ��֤�̵Ļ����ԣ���ѡ���ҩƷ��������ͭ�����⣬����Ҫ�̵�����Һ�����MnSO4��Һ���̵�����Һ��
��4��������ͼ�����ݿ�֪����������������Ϊ0.4g�����B��
�����Լ��㣺
�ٽ⣺����Ʒ��п������ΪX�����ɵ�����п������ΪY��
Zn+H2SO4=ZnSO4+H2��
65 161 2
X Y 0.4g
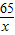 =
= ��
��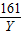 =
=
X=13.0g��Y=32.2g��
M��Cu��=20g-13g=7g��
��Ʒ��ͭ����������Ϊ��
 ×100%=35%��
×100%=35%��������п��Һ����������Ϊ��
 ×100%=20%��
×100%=20%������Ʒ��ͭ����������Ϊ35%������п��Һ����������Ϊ20%��
������������Ҫ�������ͻ�ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�

��ϰ��ϵ�д�
�����Ŀ
��2011?������һģ����ƻ��ƾ��ʲô���Ǽ̡��⣨�㣩��ݡ����������������桱֮����һ���������˵���ǽ���ƻ���۸�����ƻ���С��ǻ۹�������������������ƣ�����ά����C���������й�VC��̽����
��1��ά��������������Ӫ�����е�һ�֣�������Ӫ������______�ȣ�����2�֣�
��2��VCˮ��Һ�Ƿ�������ԣ���10ƬVCƬ��������100ml����ˮ�У��õ���ɫ����VC��Һ����������ʵ�飬����д���пո�
��3����ͬ������VC�����ıȽ��о���
�������ϣ�VC�����ˮ�еĵⷴӦ������ɫ���ʣ���ˮ����������Һ����ɫ��
ʵ����ƣ�����һ��Ũ�ȵĵ�ˮ������Һ���������Թ��и���5ml����Һ������ͬ��֭�������У��������������Һ��ɫ����Ϊ��Ӧ�յ㣮
ʵ���¼��
ʵ����ۣ�______
��˼�뽻����
������ʵ�����������飿Ŀ����______��
��ʵ������У���һЩ������ʲôҪ����______����1����
�۶�VC��һ��̽���������У���______����1�ʣ�
ʵ��Ӧ�ã�ijƷ�ƹ�֭�İ�װ���ϱ���ά����C������50mg/100mL��
��1����ѧ��ÿ����ҪԼ60mgά����C������ȫ�ӹ�֭���䣬����Ҫ��Ʒ�ƹ�֭______mL��
��2����֪��VC����Է�������Ϊ176�����Ԫ��C��H��O��������Ϊ9��1��12�����仯ѧʽΪ______��

��1��ά��������������Ӫ�����е�һ�֣�������Ӫ������______�ȣ�����2�֣�
��2��VCˮ��Һ�Ƿ�������ԣ���10ƬVCƬ��������100ml����ˮ�У��õ���ɫ����VC��Һ����������ʵ�飬����д���пո�
| ʵ�鷽�� | ʵ������ | ʵ����� |
| 1ȡ�����μ���ɫʯ����Һ | ______ | ��Һ������ |
| ��______ | �д�����ɫ�������� | ���������ʴ��� |
| ��ȡ��������������Һ���������η�̪���ٵμ�VC��Һ | ______ | ���������ʴ��� |
�������ϣ�VC�����ˮ�еĵⷴӦ������ɫ���ʣ���ˮ����������Һ����ɫ��
ʵ����ƣ�����һ��Ũ�ȵĵ�ˮ������Һ���������Թ��и���5ml����Һ������ͬ��֭�������У��������������Һ��ɫ����Ϊ��Ӧ�յ㣮
ʵ���¼��
| �������� | �μӹ�֭���� |
| ��Դ��֭ | 47 |
| ũ��� | 10 |
| �Ҵ��ζ�� | ����50�β���ɫ |
��˼�뽻����
������ʵ�����������飿Ŀ����______��
��ʵ������У���һЩ������ʲôҪ����______����1����
�۶�VC��һ��̽���������У���______����1�ʣ�
ʵ��Ӧ�ã�ijƷ�ƹ�֭�İ�װ���ϱ���ά����C������50mg/100mL��
��1����ѧ��ÿ����ҪԼ60mgά����C������ȫ�ӹ�֭���䣬����Ҫ��Ʒ�ƹ�֭______mL��
��2����֪��VC����Է�������Ϊ176�����Ԫ��C��H��O��������Ϊ9��1��12�����仯ѧʽΪ______��



