��Ŀ����
�������������������������Ź㷺��Ӧ�á�
 ������������Ʒ����Ҫ���ý����������õ����Ե��ǣ�___ ____��
������������Ʒ����Ҫ���ý����������õ����Ե��ǣ�___ ____��
A���������� B������ϸ˿ C��ͭ�Ƶ��� D.����
�ƹ�ҵ�����У��и�����ʱ������ͭ��Һ�������ϻ��߿����º�ɫ��ӡ�����йط�Ӧ�Ļ�ѧ����ʽΪ____ _ _��
����Ҫ�Ƚ������̡�ͭ�Ľ������ǿ������ѡ���ҩƷ��������ͭ�����⣬����Ҫ ��
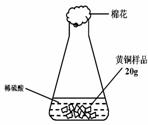
���о���ѧϰС��Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ��õ�����ƽ�ֱ�Ƶ���ƿ����������Ϊ44.1g����ȡ��ͭ
��Ʒ20.0g������ƿ�м������Ʒ������ϡ�����ƿ������������ͼ1��ʾ����������ƽ���������ݻ��ͼ2��
������������ݣ��ش��������⣺
�����ĸ�ͬѧ�Ӷ�Ƕȴ������ݣ��������ݴ�����ͼ������ȷ���� ��
�����Լ��㣺
����Ʒ��ͭ��������������ǡ�÷�Ӧʱ������Һ�����ʵ�����������

��1��A ��2��Fe + CuSO4 === Cu + FeSO4
��3��MnSO4��Һ���̵�����Һ�����������֣� ��4������B
�����Լ��㣺
�ٽ⣺����Ʒ��п������ΪX�����ɵ�����п������ΪY
Zn + H2SO4 = ZnSO4 + H2��
65 161 2
X Y 0.4g
65/X = 2/ 0.4g 161/Y =2/0.4g
X=13.0g Y=32.2g 1��
M(Cu)=20g-13g=7g
��Ʒ��Cu%=7g/20g��100%=35% 1��
�� ZnSO4 %=32.2g/(212.1g-44.1g-7g) ��100%=20%


 ��2013?��̨����ģ���������������������������Ź㷺��Ӧ�ã�
��2013?��̨����ģ���������������������������Ź㷺��Ӧ�ã�